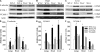Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis - PubMed (original) (raw)
Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis
Xiaobing Ye et al. J Exp Med. 2008.
Erratum in
- J Exp Med. 2008 Jun 9;205(6):1509
Abstract
To define the roles of endothelial-intrinsic nuclear factor kappaB (NF-kappaB) activity in host defense and multiple organ injury in response to sepsis, we generated double transgenic (TG) mice (EC-rtTA/I-kappaB alpha mt) that conditionally overexpress a degradation-resistant form of the NF-kappaB inhibitor I-kappaB alpha (I-kappaB alpha mt) selectively on vascular endothelium. The EC-rtTA/I-kappaB alpha mt mice had no basal, but a relatively high level of doxycycline-inducible, I-kappaB alpha mt expression. I-kappaB alpha mt expression was detected in endothelial cells, but not in fibroblasts, macrophages, and whole blood cells, confirming that transgene expression was restricted to the endothelium. When subjected to endotoxemia, EC-rtTA/I-kappaB alpha mt mice showed endothelial-selective blockade of NF-kappaB activation, repressed expression of multiple endothelial adhesion molecules, reduced neutrophil infiltration into multiple organs, decreased endothelial permeability, ameliorated multiple organ injury, reduced systemic hypotension, and abrogated intravascular coagulation. When subjected to cecal ligation and puncture-induced sepsis, the TG mice had less severe multiple organ injury and improved survival compared with wild-type (WT) mice. WT and EC-rtTA/I-kappaB alpha mt mice had comparable capacity to clear three different pathogenic bacteria. Our data demonstrate that endothelial NF-kappaB activity is an essential mediator of septic multiple organ inflammation and injury but plays little role in the host defense response to eradicate invading pathogenic bacteria.
Figures
Figure 1.
Generation of double TG EC-rtTA/I-κBαmt mice. (A) Schematic representation of VeCadrtTA and TreI-κBαmt transgenes. Transactivator (rtTA) expression is controlled by the endothelial-specific promoter VE–cadherin-5 (Ve-cad). Human I-κBαmt gene expression is controlled by a TRE-CMV fusion promoter, whose activation requires the binding of rtTA and Dox. (B and C) RT-PCR photograph showing Dox-induced I-κBαmt mRNA expression in EC-rtTA/I-κBαmt TG mice. Mouse TV616 was fed with Dox for 4 d, and mouse TV614, a transgene-positive littermate of TV616, was not fed with Dox. RT-PCR analysis detected I-κBαmt mRNA expression in 11 out of the 12 organs from mouse TV616 (B) but detected no I-κBαmt expression in any organ from mouse TV614 (C). GAPDH serves as internal control. Aot, aorta; Brn, brain; Ht, heart; Itn, intestine; Kid, kidney; Liv, liver; Lug, lungs; M, DNA marker; N, negative control; P, positive control; Skm, skeletal muscle; Spl, spleen; Stam, stomach; Thyd, thyroid; Ton, tongue. (D–F) Immunofluorescence staining for Dox-induced I-κBαmt protein expression in lung sections of EC-rtTA/I-κBαmt mice. (D) Dox+ mice, preimmune IgG, no staining. (E) Dox− mice, anti–human I-κBα, weak staining (endogenous mouse I-κBα). (F) Dox+ mice, anti–human I-κBα, stronger staining (Dox-induced I-κBαmt protein). Bars, 100 μm.
Figure 2.
Endothelial-selective I-κBαmt protein expression in EC-rtTA/I-κBαmt mice. (A) TG, but not WT, ECs express Dox-induced I-κBαmt protein. WT ECs were untreated (WT-C) or stimulated with TNF-α (WT-T) for 15 min. TG ECs were untreated (TG0) or treated with 0.5 μg/ml (TG0.5) or 1 μg/ml (TG1) Dox for 48 h to induce I-κBαmt expression, and stimulated with TNF-α for 15 min. Western blotting using anti–I-κBα antibody detected a single band on cytoplasmic protein from WT ECs, and two bands on cytoplasmic protein from TG ECs, one representing endogenous mouse I-κBα (MI-κBα) and the other representing the transgene product human I-κBαmt (HI-κBαmt). The HI-κBαmt band was weak in cells without Dox incubation (TG0), but its intensity increased with increasing doses of Dox (TG0.5 and TG1). The MI-κBα band was diminished by TNF-α treatment, indicating degradation, whereas the HI-κBαmt band was not affected by TNF-α stimulation, indicating degradation resistance. (B) TG fibroblasts express no Dox-induced I-κBαmt protein. Fibroblasts were isolated from the same lungs where ECs were isolated, cultured, and treated with Dox and TNF-α in an identical way as in ECs. Western blotting detected a single MI-κBα band, which was diminished by TNF-α treatment, but detected no HI-κBαmt band. TG-C, untreated TG cells; TG-T, TNF-α–treated TG cells. (C) TG macrophages express no Dox-induced I-κBαmt protein. TG macrophages were treated with Dox to induce I-κBαmt expression. Cells were unstimulated (WT-C and TG-C) or stimulated with LPS (WT-L and TG-L) for 30 min. Western blotting detected a single MI-κBα band, which was diminished by LPS treatment, but detected no Dox-induced HI-κBαmt band. Membranes for I-κBα were reblotted with actin antibody (Actin).
Figure 3.
I-κBαmt mRNA expression in whole blood cells of EC-rtTA/I-κBαmt mice. Mice 1 and 2 (Ms1 and Ms2) were not fed with Dox, and mice 3–5 (Ms3, Ms4, and Ms5) were fed with Dox for 4 d. RT-PCR detected no I-κBαmt expression in whole blood cells and lungs of Ms1 and Ms2 (without Dox), and detected a strong I-κBαmt band in lungs but not in whole blood cells of Ms3, Ms4, and Ms5 (with Dox). GAPDH serves as internal control. Bld, whole blood cells; Lug, lungs; M, DNA marker; P, positive control.
Figure 4.
Endothelial-selective blockade of NF-κB activation in EC-rtTA/I-κBαmt mice. We used p65 nuclear translocation as an indicator of NF-κB activation. All cells were treated with Dox for 48 h to avoid the Dox effect. Control cells (WT-C and TG-C) were untreated. Treated ECs and fibroblasts were stimulated with TNF-α (WT-T and TG-T) for 15 min, and macrophages were stimulated with LPS (WT-L and TG-L) for 30 min. Western blotting detected a strong p65 band in nuclear proteins from all three WT cells stimulated with TNF-α or LPS (WT-T and WT-L), indicating NF-κB activation. The TNF-α– or LPS-induced p65 band was abrogated in TG ECs (A, TG-T) but not in TG fibroblasts and TG macrophages (B and C, TG-T and TG-L), indicating EC-selective blockade of NF-κB activation. Membranes for p65 were reblotted with actin antibody (Actin).
Figure 5.
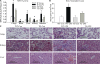
Repressed expression of adhesion molecules in EC-rtTA/I-κBαmt mice. (A–C) Western blot photographs showing levels of E-selectin, ICAM-1, and VCAM-1 protein expression in the lungs (A), heart (B), and liver (C) of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice. Membrane for E-selectin, ICAM-1, or VCAM-1 blotting was reblotted with actin antibody (Actin). (D–F) Densitometry quantification of E-selectin, ICAM-1, and VCAM-1 bands. Tissue levels of the three adhesion molecules were similar between WT-Con and TG-Con mice. Compared with WT-LPS mice, the LPS-induced E-selectin, ICAM-1, and VCAM-1 protein expression was significantly reduced in all three organs of TG-LPS mice. The means ± SEM of five animals are shown. *, P < 0.05 compared with the other three groups.
Figure 6.
Reduced neutrophil infiltration and alleviated organ injury in endotoxemic EC-rtTA/I-κBαmt mice. (A) MPO activity in the lungs, heart, liver, kidney, and intestine of WT and TG mice treated with saline or LPS for 4 h. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a marked increase in MPO activity in all five organs, which was significantly reduced in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Neutrophil counts in BAL fluids. Compared with WT-Con and TG-Con mice, WT-LPS mice had a marked increase in BAL fluid neutrophil count, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) Histopathological evaluation of PMN infiltration and organ injury. Paraffin-embedded sections were prepared from the lungs, kidney, and liver of WT and TG mice treated with saline or LPS for 5 h, and subjected to hematoxylin and eosin staining. Lungs of WT-Con and TG-Con mice show thin alveolar wall and normal cellularity (C1 and C2). The lung of a WT-LPS mouse shows intense cellular infiltration, alveolar septal wall thickness, interstitial edema, and alveolar congestion (C3). Kidneys of WT-Con and TG-Con mice show normal histological structures of cortex with glomerulus (C5 and C6). The kidney of a WT-LPS mouse shows increased cellular infiltration, glomerular and sinusoidal congestion, and signs of tubular swelling and cell injury (C7). Livers of WT-Con and TG-Con mice show a normal histological appearance of the central vein surrounded by hepatocytes and sinusoids (C9 and C10). The liver of a WT-LPS mouse shows a congested central vein, hemorrhage, and signs of hepatic cell injury and necrosis (C11). The pathological changes caused by LPS were significantly alleviated in TG-LPS mice in all three organs (C4, C8, and C12). Bars, 100 μm.
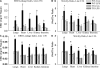
Figure 7.
Reduced endothelial permeability and organ edema in endotoxemic EC-rtTA/I-κBαmt mice. (A) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 5 h after LPS injection. Compared with WT-Con and TG-Con mice, WT-LPS mice had a marked increase in EBD leakage index in all five organs, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 5 h after LPS injection. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a significantly increased tissue wet/dry ratio in all five organs, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 1/36 TG mice 5 h after LPS injection. WT-LPS mice had a marked increase in EBD leakage index in all five organs, which was reversed in TG-LPS mice. The means ± SEM of six animals are shown. *, P < 0.05 compared with the other three groups. (D) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 1/36 TG mice 5 h after LPS injection. WT-LPS mice showed a significantly increased tissue wet/dry ratio in all five organs, which was reversed in TG-LPS mice. The means ± SEM of six animals are shown. *, P < 0.05 compared with the other three groups.
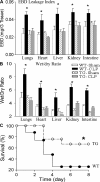
Figure 8.
Reduced organ injury and improved survival in septic EC-rtTA/I-κBαmt mice. (A) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 24 h after sham or CLP operation. Compared with WT-sham and TG-sham mice, WT-CLP mice showed a marked increase in EBD leakage index in all five organs, which was reversed in TG-CLP mice. The means ± SEM of eight animals are shown. *, P < 0.05 compared with the other three groups. (B) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice. Compared with WT-sham and TG-sham mice, WT-CLP mice showed a significantly increased tissue wet/dry ratio, which was reversed in TG-CLP mice. The means ± SEM of eight animals are shown. *, P < 0.05 compared with the other three groups. (C) Survival of WT-CLP and TG-CLP mice. Animals were subjected to CLP and followed for 14 d (no further mortality after 8 d). Compared with WT mice, TG mice showed a significantly improved survival. *, P < 0.0003 compared with WT mice using the log-rank test (21 mice per group).
Figure 9.
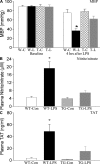
Reduced systemic hypotension and coagulation in endotoxemic EC-rtTA/I-κBαmt mice. (A) Systemic MBP was monitored before (baseline) and at 4 h after LPS challenge. Baseline MBP was identical among the four groups of mice. Compared with WT-Con (W-C) and TG-Con (T-C) mice, WT-LPS (W-L) mice showed a significant drop in MBP at 4 h after LPS, which was reduced in TG-LPS (T-L) mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Plasma concentration of nitrite/nitrate in WT and EC-rtTA/I-κBαmt mice. Plasma levels of nitrite/nitrate were low in WT-Con and TG-Con mice, markedly elevated in WT-LPS mice, and significantly reduced in TG-LPS mice, as compared with WT-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) Plasma levels of TAT complex in WT and EC-rtTA/I-κBαmt mice. The plasma level of TAT, an indicator of coagulation, was very low in WT-Con and TG-Con mice but markedly elevated in WT-LPS mice, and reduced significantly in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups.
Figure 10.
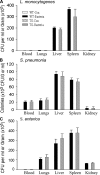
EC-rtTA/I-κBαmt mice preserve bacterial clearance capacity. WT and TG mice were injected with saline or pathogenic bacteria. Bacterial colonies that formed in blood and tissue homogenate cultures were counted and expressed as CFU per milliliter of blood or per gram of tissue. (A) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 24 h after i.v. injection of 108 CFU of L. monocytogenes. No bacteria grew in the blood and any tissue homogenate from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown. (B) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 12 h after i.v. injection of 107 CFU of S. pneumoniae. No bacteria grew in the blood and any tissue homogenate from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown. (C) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 12 h after i.v. injection of 108 CFU of S. enterica. No bacteria grew in the blood and tissue homogenates from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown.
Similar articles
- A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction.
Ding J, Song D, Ye X, Liu SF. Ding J, et al. J Immunol. 2009 Sep 15;183(6):4031-8. doi: 10.4049/jimmunol.0900105. Epub 2009 Aug 19. J Immunol. 2009. PMID: 19692637 Free PMC article. - Activation of endothelial intrinsic NF-{kappa}B pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice.
Song D, Ye X, Xu H, Liu SF. Song D, et al. Blood. 2009 Sep 17;114(12):2521-9. doi: 10.1182/blood-2009-02-205914. Epub 2009 Jul 20. Blood. 2009. PMID: 19620400 Free PMC article. - NF-kappa B activation as a pathological mechanism of septic shock and inflammation.
Liu SF, Malik AB. Liu SF, et al. Am J Physiol Lung Cell Mol Physiol. 2006 Apr;290(4):L622-L645. doi: 10.1152/ajplung.00477.2005. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16531564 Review. - Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis.
Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J, Petzelbauer P, Assinger A, Schmid JA. Mussbacher M, et al. Front Immunol. 2019 Feb 4;10:85. doi: 10.3389/fimmu.2019.00085. eCollection 2019. Front Immunol. 2019. PMID: 30778349 Free PMC article. Review.
Cited by
- An in vitro iron superoxide dismutase inhibitor decreases the parasitemia levels of Trypanosoma cruzi in BALB/c mouse model during acute phase.
Olmo F, Urbanová K, Rosales MJ, Martín-Escolano R, Sánchez-Moreno M, Marín C. Olmo F, et al. Int J Parasitol Drugs Drug Resist. 2015 Jun 20;5(3):110-6. doi: 10.1016/j.ijpddr.2015.05.002. eCollection 2015 Dec. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26236582 Free PMC article. - Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases.
Clark PR, Kim RK, Pober JS, Kluger MS. Clark PR, et al. PLoS One. 2015 Mar 27;10(3):e0120075. doi: 10.1371/journal.pone.0120075. eCollection 2015. PLoS One. 2015. PMID: 25816133 Free PMC article. - Inflection points in sepsis biology: from local defense to systemic organ injury.
Seeley EJ, Matthay MA, Wolters PJ. Seeley EJ, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Sep;303(5):L355-63. doi: 10.1152/ajplung.00069.2012. Epub 2012 Jun 15. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22707617 Free PMC article. Review. - Identification of Aryl Polyamines Derivatives as Anti-Trypanosoma cruzi Agents Targeting Iron Superoxide Dismutase.
Martín-Escolano R, Molina-Carreño D, Martín-Escolano J, Clares MP, Galiana-Roselló C, González-García J, Cirauqui N, Llinares JM, Rosales MJ, García-España E, Marín C. Martín-Escolano R, et al. Pharmaceutics. 2022 Dec 31;15(1):140. doi: 10.3390/pharmaceutics15010140. Pharmaceutics. 2022. PMID: 36678771 Free PMC article. - Up-regulation of DcR3 in microbial toxins-stimulated HUVECs involves NF-κB signalling.
Hou Y, Liang D, Liu Y, Chen H, Lou X. Hou Y, et al. BMC Biochem. 2018 Dec 27;19(1):13. doi: 10.1186/s12858-018-0102-z. BMC Biochem. 2018. PMID: 30587127 Free PMC article.
References
- Martin, G.S., D.M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554. - PubMed
- Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. - PubMed
- Liu, S.F., and A.B. Malik. 2006. NF-κB activation as a pathologic mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L622–L645. - PubMed
- Brown, M.A., and W.K. Jones. 2004. NF-kappaB action in sepsis: the innate immune system and the heart. Front. Biosci. 9:1201–1217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous