Genetic basis for kidney cancer: opportunity for disease-specific approaches to therapy - PubMed (original) (raw)
Review
Genetic basis for kidney cancer: opportunity for disease-specific approaches to therapy
Elizabeth Cartwright Pfaffenroth et al. Expert Opin Biol Ther. 2008 Jun.
Abstract
Background: Kidney cancer is not a homogenous entity; it is comprised of many different tumor types, with different biologies and molecular mechanisms leading to disease and therefore different treatment approaches.
Objective: To describe the genetic basis and biochemical pathways underlying inherited forms of renal cancer, specifically in four described syndromes (von Hippel-Lindau [VHL], hereditary papillary renal cancer [HPRC], Birt-Hogg-Dubé [BHD] and hereditary leiomyomatosis renal cell carcinoma [HLRCC]), and to elucidate how the understanding of these diseases enables the possibility of disease-specific approaches to therapy.
Methods: A systematic review of the published literature on inherited and sporadic forms of renal cancer was performed.
Conclusion: Understanding of the biology and mechanisms of different forms of kidney cancer provides an opportunity for development of new treatment options.
Figures
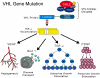
Figure 1
Kidney cancer is not a single disease; it is made up of a number of different types of cancer that occur in the kidney, each with different histologic types, having a different clinical course, and associated with alteration of a different gene. Percentages represent the frequency of the renal carcinoma subtype. (From LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172.)[2]
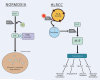
Figure 2
Molecular targeting of the VHL pathway in clear cell renal carcinoma: Mutation of the VHL gene in clear cell kidney cancer results in increased accumulation of HIF and the resulting increase in transcription of downstream targets such as VEGF, Glut-1, and TGFα (A). Mutation of the VHL gene in the α domain (demonstrated here) inhibits binding to elongin C and formation of the VHL complex.[31] Mutations in other parts of the gene, such as the β domain, prevents binding to and ubiquitin mediated degradation of HIF.[32] Potential disease-specific therapeutic approaches include agents which block the function of HIF (B), VEGFR, or EGFR (C). (From LINEHAN WM, ZBAR B: Focus on kidney cancer. Cancer cell (2004) 6(3):223-228.)[35]
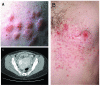
Figure 3
Non-renal manifestations of BHD: (A-D) Multiple fibrofolliculomas and (E) multiple pulmonary cysts. (Adapted from LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172. and TORO JR, GLENN G, DURAY P et al.: Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Archives of dermatology (1999) 135(10):1195-1202.)[2,55]
Figure 4
Non-renal clinical manifestations of HLRCC: (A,B) cutaneous leiomyomas in a grouped and segmental distribution, (C) multiple large uterine fibroids in a female patient. (Adapted from TORO JR, NICKERSON ML, WEI MH et al.: Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. American journal of human genetics (2003) 73(1):95-106 and LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172.)[2,69]
Figure 5
Normoxia: In the presence of oxygen (as a substrate), HIF is hydroxylated by HPH, which allows the VHL complex to recognize and target it for ubiquitin-mediated degradation in the proteosome. HLRCC: Loss of FH shunts the TCA cycle to produce excess fumarate. Fumarate stabilizes HIF through competitive inhibition of HPH, allowing HIF to remain unhydroxylated and thus avoiding degredation. Elevated HIF drives transcription products involved with angiogenesis (VEGF), glucose transport (GLUT-1), and growth stimulation (TGF-α, PDGF).
Similar articles
- Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management.
Maher ER. Maher ER. World J Urol. 2018 Dec;36(12):1891-1898. doi: 10.1007/s00345-018-2288-5. Epub 2018 Apr 21. World J Urol. 2018. PMID: 29680948 Free PMC article. Review. - The genetic basis of cancer of the kidney.
Linehan WM, Walther MM, Zbar B. Linehan WM, et al. J Urol. 2003 Dec;170(6 Pt 1):2163-72. doi: 10.1097/01.ju.0000096060.92397.ed. J Urol. 2003. PMID: 14634372 Review. - Hereditary kidney cancer: unique opportunity for disease-based therapy.
Linehan WM, Pinto PA, Bratslavsky G, Pfaffenroth E, Merino M, Vocke CD, Toro JR, Bottaro D, Neckers L, Schmidt LS, Srinivasan R. Linehan WM, et al. Cancer. 2009 May 15;115(10 Suppl):2252-61. doi: 10.1002/cncr.24230. Cancer. 2009. PMID: 19402075 Free PMC article. Review. - The clinical implications of the genetics of renal cell carcinoma.
Rosner I, Bratslavsky G, Pinto PA, Linehan WM. Rosner I, et al. Urol Oncol. 2009 Mar-Apr;27(2):131-6. doi: 10.1016/j.urolonc.2008.11.001. Urol Oncol. 2009. PMID: 19285230 Free PMC article. Review. - Treatment Strategies for Hereditary Kidney Cancer: Current Recommendations and Updates.
Singh S, Chaurasia A, Gopal N, Malayeri A, Ball MW. Singh S, et al. Discov Med. 2022 Nov-Dec;34(173):205-220. Discov Med. 2022. PMID: 36602871 Review.
Cited by
- SENP1 promotes proliferation of clear cell renal cell carcinoma through activation of glycolysis.
Dong B, Gao Y, Kang X, Gao H, Zhang J, Guo H, You MJ, Xue W, Cheng J, Huang Y. Dong B, et al. Oncotarget. 2016 Dec 6;7(49):80435-80449. doi: 10.18632/oncotarget.12606. Oncotarget. 2016. PMID: 27741516 Free PMC article. - Targeted therapies for non-clear renal cell carcinoma.
Singer EA, Bratslavsky G, Linehan WM, Srinivasan R. Singer EA, et al. Target Oncol. 2010 Jun;5(2):119-29. doi: 10.1007/s11523-010-0148-3. Epub 2010 Aug 3. Target Oncol. 2010. PMID: 20680492 Free PMC article. Review. - Contemporary treatment of metastatic renal cell carcinoma.
Wiechno P, Kucharz J, Sadowska M, Michalski W, Sikora-Kupis B, Jonska-Gmyrek J, Poniatowska G, Nietupski K, Ossolinski K, Demkow T. Wiechno P, et al. Med Oncol. 2018 Oct 27;35(12):156. doi: 10.1007/s12032-018-1217-1. Med Oncol. 2018. PMID: 30368624 Review. - Renal Carcinogenesis, Tumor Heterogeneity, and Reactive Oxygen Species: Tactics Evolved.
Shanmugasundaram K, Block K. Shanmugasundaram K, et al. Antioxid Redox Signal. 2016 Oct 20;25(12):685-701. doi: 10.1089/ars.2015.6569. Epub 2016 Jul 27. Antioxid Redox Signal. 2016. PMID: 27287984 Free PMC article. Review. - Pattern of care and treatment outcomes of metastatic non-clear cell kidney cancer: a single centre experience from India.
Roy S, Raychaudhuri S, Biswas B, Dabkara D, Bhattacherjee A, Ganguly S, Ghosh J, Patel YS, Pal S, Karmakar J, Mitra A, Gupta S. Roy S, et al. Ecancermedicalscience. 2024 Sep 23;18:1775. doi: 10.3332/ecancer.2024.1775. eCollection 2024. Ecancermedicalscience. 2024. PMID: 39430082 Free PMC article.
References
- JEMAL A, SIEGEL R, WARD E, MURRAY T, XU J, SMIGAL C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
- LINEHAN WM, WALTHER MM, ZBAR B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–2172. - PubMed
- GNARRA JR, TORY K, WENG Y, SCHMIDT L, WEI MH, LI H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Gen. 1994;7:85–90. - PubMed
- SHUIN T, KONDO K, TORIGOE S, KISHIDA T, KUBOTA Y, HOSAKA M, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–2855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous