Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1 - PubMed (original) (raw)
Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1
Shigeko Yamashiro et al. Dev Cell. 2008 May.
Abstract
Myosin phosphatase-targeting subunit 1 (MYPT1) binds to the catalytic subunit of protein phosphatase 1 (PP1C). This binding is believed to target PP1C to specific substrates including myosin II, thus controlling cellular contractility. Surprisingly, we found that during mitosis, mammalian MYPT1 binds to polo-like kinase 1 (PLK1). MYPT1 is phosphorylated during mitosis by proline-directed kinases including cdc2, which generates the binding motif for the polo box domain of PLK1. Depletion of PLK1 by small interfering RNAs is known to result in loss of gamma-tubulin recruitment to the centrosomes, blocking centrosome maturation and leading to mitotic arrest. We found that codepletion of MYPT1 and PLK1 reinstates gamma-tubulin at the centrosomes, rescuing the mitotic arrest. MYPT1 depletion increases phosphorylation of PLK1 at its activating site (Thr210) in vivo, explaining, at least in part, the rescue phenotype by codepletion. Taken together, our results identify a previously unrecognized role for MYPT1 in regulating mitosis by antagonizing PLK1.
Figures
Fig. 1
Identification of the mitosis-specific phosphorylation sites of MYPT1. A, Phosphopeptide map analyses of wild-type (a), as well as single point mutants of S432A (b), S473A (c), S601A (d) and a triple mutant (e). M2/3 spot disappeared by S432A (Ser432 replaced with Ala) mutation (b), M5 by S473A mutation (c); M1 by S601 mutation (d). M1, M2/3 and M5 all disappeared by triple mutation (e). X is a Xenopus specific spot. The directions of the first dimension (electrophoresis, EP) and the second dimension (thin layer chromatography, TLC) are indicated by arrows. B, In vitro phosphorylation of MYPT1 by cdc2. Upper panel, autoradiography; lower panel, Coomassie brilliant blue staining. C, Phosphopeptide map analyses of MYPT1 phosphorylated in vivo (a) and phosphorylated in vitro by cdc2 (b). Phosphorylation at the M2/3 site sometimes gave two spots as shown here (Totsukawa et al., 1999). D, diagram of rat MYPT1 indicating locations of the mitosis-specific phosphorylation sites.
Fig. 2
Phosphorylation-dependent association of MYPT1 and PLK1. A, Polo-box domain binding motif and sequence alignment of various vertebrate MYPT1s at Ser473. B, Co-immunoprecipitation of MYPT1 and PLK1. I, interphase (lanes 1 & 3); M, mitosis (lanes 2, 4, 5, 6). MYPT1 immunoprecipitates (IP) (lane 3 and 4) were blotted with anti-MYPT1, PLK1, PP1C and aurora B antibodies. PLK1 IP (lane 5) was blotted with anti-MYPT1, PP1C and PLK1 antibodies. EB1 immunoprecipitates (lane 6) as a negative control. C, Dot blot analyses. Binding of the GST-tagged PBD or mutant PBD to phosphopeptides was detected by an anti-GST antibody. D, GST pull-down assay. The central domain (388–636) of MYPT (lanes 4 & 5) and its mutant (Ser473A, lane 6) were phosphorylated with (lanes 5 & 6) or without (lane 4) cdc2, and their binding to GST-PBD was examined by Western blotting. Lanes 1–3, GST alone as a control. E, Far-Western ligand binding assay. Binding of GST-PBD to unphosphorylated, control MYPT1 (lane 1) and cdc2-phosphorylated MYPT1 (lane 2) was detected with a GST antibody (upper panel). Equal loading of MYPT1 was confirmed by Western blotting (lower panel). F, Western blot analyses of an antibody against S473-phosphorylated MYPT1 (pS473-Ab, lanes 1 & 2). M, mitosis; I, interphase. Lanes 3 & 4, Western blot with a general MYPT1 antibody.
Fig. 3
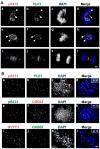
Immunofluorescent localization of S473-phosphorylated MYPT1 during mitosis. A, HeLa cells at prophase (a–d), prometaphase (e–h) or telophase (i–l) were stained with the anti-S473-phosphorylated MYPT1 antibody (pS473) and an anti-PLK1 antibody. Bar, 5μm. B, localization of phosphorylated MYPT1 at the kinetochores. Mitotic chromosome spreads were stained with pS473 and PLK1 antibodies (a–d) or pS473 and CREST antibodies (e–h) or MYPT1 and CREST antibodies (i–l).
Fig. 4
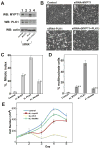
MYPT1 depletion rescues mitotic arrest caused by PLK1 depletion. A, Western blot analyses to detect levels of MYPT1 and PLK1 of control (lane 1), MYPT1-depleted (lane 2), PLK1-depleted (lane 3) or both MYPT1- and PLK1-depelted (lane 4) SW962 cells. B, Phase-contrast images of control (a), MYPT1-depleted (b), PLK1-depleted (c), and double-depleted (d) SW962 cells. C, Mitotic indexes of control, MYPT1-depleted, PLK1-depleted, double-depleted cells (200–300 cells were counted, repeated three times). D, Percentage of multinucleate cells in control, MYPT1-depleted, PLK1-depleted, and double-depleted cells (400–500 cells were counted, repeated three times). E, Cell proliferation analyses of control, MYPT1-depleted, PLK1-depleted, and double-depleted HeLa cells. HeLa cells were transfected at Day1 with siRNA. Note that about two-thirds of PLK1-depleted cells at Day2 were mitotically arrested. Error bars for C, D & E are standard deviation.
Fig. 5
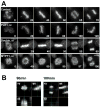
Live cell imaging of control, PLK1-depleted, double-depleted and MYPT1-depleted cells. A, HeLa cells stably expressing histone H2B-GFP were transfected with siRNAs to deplete PLK1, MYPT1 or both and imaged using a DeltaVision Image Restoration Microscope system. Five Z-section images were taken in 1min intervals, deconvoluted, and projected images were shown. Bar, 10μm. Time at minutes. B, orthogonal images of MYPT1-depleted cells at 90min and 100min.
Fig. 6
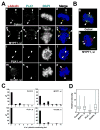
Double depletion of MYPT1 and PLK1 reinstates γ-tubulin at centrosomes. A, Localization of γ-tubulin (red), PLK1 (green) and DNA (blue) in control (a–d), MYPT1-depleted (e–h), PLK1-depleted (i–l), and double-depleted (m–p) SW962 cells. Arrowheads indicate centrosomes. Bar, 5μm. B, Localization of γ-tubulin (red), MYPT1 (green) and DNA (blue) in control (a) and MYPT1-depleted (b) cells. C, Distributions of the number of γ-tubulin-containing foci per cell for control, MYPT1-depleted, PLK1-depleted, and both MYPT1- and PLK1-depleted mitotic SW962 cells. D, Box plot of γ-tubulin intensities of individual centrosomes of control, MYPT1-depleted, PLK1-depleted, and double-depleted cells. The upper and lower edge of each box represent upper and lower quartilies, respectively. The median indicated by a horizontal line.
Fig. 7
Restoration of Cdc25c phosphorylation by double depletion and increase in Thr210 phosphorylation by MYPT1 depletion. A, Cdc25c phosphorylation in control (lane 3), MYPT1-depleted (lane 4), PLK1-depleted (lane 5), and double-depleted (lane 6), mitotic cells was immunoblotted with the anti-Cdc25c antibody. The mobility of interphase Cdc25c is shown in lanes 1 for comparison with that of nocodazole arrested, mitotic Cdc25c (lane 2). B, double label immunofluorescence of control and MYPT1-depleted cells stained with anti-phospho-Thr210 (red) and anti-PLK (green) antibodies. C, Box-plot to reveal an increase in Thr210 phosphorylation by MYPT1 depletion. Ratios (pThr210/PLK1) of staining intensities on centrosomes (n=52 for control, n=88 for depleted cells) were analyzed. D, Western blot of total lysates from control and MYPT1-depleted cells. E, Western blot of PLK immunoprecipitates from control and MYPT1-depleted cells. Kinase activities were assayed by autophosphorylation of PLK1 (panel ‘Auto-P’) or phosphorylation of casein (panel “casein-P”). F, In vitro phosphatase assay by MYPT1/PP1. Baculovirus-expressed PLK1 (20ng) was incubated at 30oC for 20min without (lane 1) or with 8ng of MYPT1/PP1 (lane 2), and blotted with the anti-phosphoThr210 antibody.
Similar articles
- Chk2-dependent phosphorylation of myosin phosphatase targeting subunit 1 (MYPT1) regulates centrosome maturation.
Nai S, Shi Y, Ru H, Ding Y, Geng Q, Li Z, Dong MQ, Xu X, Li J. Nai S, et al. Cell Cycle. 2019 Oct;18(20):2651-2659. doi: 10.1080/15384101.2019.1654795. Epub 2019 Aug 15. Cell Cycle. 2019. PMID: 31416392 Free PMC article. - _O_-GlcNAcylation of myosin phosphatase targeting subunit 1 (MYPT1) dictates timely disjunction of centrosomes.
Liu C, Shi Y, Li J, Liu X, Xiahou Z, Tan Z, Chen X, Li J. Liu C, et al. J Biol Chem. 2020 May 22;295(21):7341-7349. doi: 10.1074/jbc.RA119.012401. Epub 2020 Apr 15. J Biol Chem. 2020. PMID: 32295844 Free PMC article. - Chk1 modulates the interaction between myosin phosphatase targeting protein 1 (MYPT1) and protein phosphatase 1cβ (PP1cβ).
Hu X, Li Z, Ding Y, Geng Q, Xiahou Z, Ru H, Dong MQ, Xu X, Li J. Hu X, et al. Cell Cycle. 2018;17(4):421-427. doi: 10.1080/15384101.2017.1418235. Epub 2018 Mar 19. Cell Cycle. 2018. PMID: 29262732 Free PMC article. - Regulating a key mitotic regulator, polo-like kinase 1 (PLK1).
Colicino EG, Hehnly H. Colicino EG, et al. Cytoskeleton (Hoboken). 2018 Nov;75(11):481-494. doi: 10.1002/cm.21504. Epub 2018 Dec 7. Cytoskeleton (Hoboken). 2018. PMID: 30414309 Free PMC article. Review. - Mechanisms underlying Plk1 polo-box domain-mediated biological processes and their physiological significance.
Lee KS, Park JE, Kang YH, Kim TS, Bang JK. Lee KS, et al. Mol Cells. 2014 Apr;37(4):286-94. doi: 10.14348/molcells.2014.0002. Epub 2014 Apr 7. Mol Cells. 2014. PMID: 24722413 Free PMC article. Review.
Cited by
- Aurora B-dependent phosphorylation of Ataxin-10 promotes the interaction between Ataxin-10 and Plk1 in cytokinesis.
Tian J, Tian C, Ding Y, Li Z, Geng Q, Xiahou Z, Wang J, Hou W, Liao J, Dong MQ, Xu X, Li J. Tian J, et al. Sci Rep. 2015 Feb 10;5:8360. doi: 10.1038/srep08360. Sci Rep. 2015. PMID: 25666058 Free PMC article. - Functional specialization of chordate CDK1 paralogs during oogenic meiosis.
Øvrebø JI, Campsteijn C, Kourtesis I, Hausen H, Raasholm M, Thompson EM. Øvrebø JI, et al. Cell Cycle. 2015;14(6):880-93. doi: 10.1080/15384101.2015.1006000. Cell Cycle. 2015. PMID: 25714331 Free PMC article. - PLK1 and its substrate MISP facilitate intrahepatic cholangiocarcinoma progression by promoting lymphatic invasion and impairing E-cadherin adherens junctions.
Pan YR, Lai JC, Huang WK, Peng PH, Jung SM, Lin SH, Chen CP, Wu CE, Hung TH, Yu AL, Wu KJ, Yeh CN. Pan YR, et al. Cancer Gene Ther. 2024 Feb;31(2):322-333. doi: 10.1038/s41417-023-00705-z. Epub 2023 Dec 6. Cancer Gene Ther. 2024. PMID: 38057358 Free PMC article. - Rebalancing of actomyosin contractility enables mammary tumor formation upon loss of E-cadherin.
Schipper K, Seinstra D, Paulien Drenth A, van der Burg E, Ramovs V, Sonnenberg A, van Rheenen J, Nethe M, Jonkers J. Schipper K, et al. Nat Commun. 2019 Aug 23;10(1):3800. doi: 10.1038/s41467-019-11716-6. Nat Commun. 2019. PMID: 31444332 Free PMC article. - Integrated analysis of CRLF2 signaling in acute lymphoblastic leukemia identifies Polo-like kinase 1 as a potential therapeutic target.
Huang TC, Cutler J, Bharne S, Zhong J, Weinstock D, Tyner J, Gojo I, Civin C, Pandey A. Huang TC, et al. Leuk Lymphoma. 2015 May;56(5):1524-7. doi: 10.3109/10428194.2014.963076. Epub 2014 Nov 5. Leuk Lymphoma. 2015. PMID: 25213184 Free PMC article. No abstract available.
References
- Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, Stukenberg PT, Gorbsky GJ. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol. 2005;15:1078–1089. - PubMed
- Axton JM, Dombradi V, Cohen PT, Glover DM. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990;63:33–46. - PubMed
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. - PubMed
- Blagden SP, Glover DM. Polar expeditions--provisioning the centrosome for mitosis. Nat Cell Biol. 2003;5:505–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL23615/HL/NHLBI NIH HHS/United States
- R01 HL023615/HL/NHLBI NIH HHS/United States
- R37 CA042742/CA/NCI NIH HHS/United States
- R01 CA042742/CA/NCI NIH HHS/United States
- CA42742/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous