Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation - PubMed (original) (raw)
. 2008 May 20;105(20):7327-32.
doi: 10.1073/pnas.0802545105. Epub 2008 May 14.
Ingrid Flores, Hong Zhang, Rui Yu, Agnieszka Staniszewski, Emmanuel Planel, Mathieu Herman, Lingling Ho, Robert Kreber, Lawrence S Honig, Barry Ganetzky, Karen Duff, Ottavio Arancio, Scott A Small
Affiliations
- PMID: 18480253
- PMCID: PMC2386077
- DOI: 10.1073/pnas.0802545105
Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation
Alim Muhammad et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Although deficiencies in the retromer sorting pathway have been linked to late-onset Alzheimer's disease, whether these deficiencies underlie the disease remains unknown. Here we characterized two genetically modified animal models to test separate but related questions about the effects that retromer deficiency has on the brain. First, testing for cognitive defects, we investigated retromer-deficient mice and found that they develop hippocampal-dependent memory and synaptic dysfunction, which was associated with elevations in endogenous Abeta peptide. Second, testing for neurodegeneration and amyloid deposits, we investigated retromer-deficient flies expressing human wild-type amyloid precursor protein (APP) and human beta-site APP-cleaving enzyme (BACE) and found that they develop neuronal loss and human Abeta aggregates. By recapitulating features of the disease, these animal models suggest that retromer deficiency observed in late-onset Alzheimer's disease can contribute to disease pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
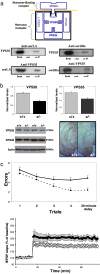
Retromer deficiency causes hippocampal-dependent memory and synaptic dysfunction. (a) (Top) The retromer sorting pathway, as first described in yeast, is made up of a multimeric retromer complex, including VPS35 and VPS26, and a retromer-binding receptor, VPS10. (Middle and Bottom) SorLA and sortilin are VPS10-containing receptors that coimmunoprecipitate with VPS35 in the mouse brain. hom, homogenate; cont, no primary antibody control; co-IP, coimmunoprecipate. (b) Retromer-deficient mice, VPS26 heterozygote KO mice (+/−), have diminished brain levels of VPS26 and VPS35, as shown in bar graphs representing mean levels (normalized to actin) measured from the brains of five +/− and five wild-type (+/+) mice. Immunoblots (Lower Left) and immunocytochemistry (Lower Right) are shown for individual examples. (c) Retromer-deficient mice have defects in hippocampal-dependent memory, as measured in the radial-arm water maze (Upper). The mean number of errors (y axis) are shown for retromer-deficient mice (solid line, +/−) and for wild-type littermates (stippled line, +/+) measured every 90 s during the first four trials and then after a 30-min delay. Retromer-deficient mice have defects in long-term potentiation (Lower), recorded from acute hippocampal slices (filled circles, +/−; open circles, +/+).
Fig. 2.
Retromer deficiency elevates levels of endogenous Aβ in the mouse brain. (a) Retromer-deficient mice (+/−) have elevated levels of endogenous Aβ40 and Aβ42 (Upper, measured in 16 +/− and 18 +/+ mice). Retromer-deficient mice (+/−) have elevated levels of endogenous sAPPβ (Lower Left). Levels of sAPPβ and Aβ are significantly correlated (Lower Right). (b) Immunoblots from the brains of three retromer-deficient mice (+/−) and two wild-type littermates (+/+) show that, except for sAPPβ, no differences were observed for full-length APP, CTFs, or sAPPα. (c) Administration of the γ-secretase inhibitor L-685,458 rescues the LTP defects in retromer-deficient mice (filled circles, +/−; open circles, +/+).
Fig. 3.
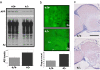
Retromer deficiency elevates levels of human Aβ in the brains of flies expressing human APP and BACE and causes neurodegeneration. (a) Retromer-deficient flies (+/− for VPS35) expressing human APP and human BACE have elevated brain levels of human Aβ compared with control flies (+/+ for VPS35) expressing human APP and human BACE. Immunoblots in Upper show individual examples (note reduction in full-length APP), and the bar graph in Lower shows means levels from 10 flies per genotype. (b) High-magnification view of frontal brain sections at approximately midbrain from control (+/+) and retromer-deficient (+/−) flies expressing human APP and human BACE stained with 4G8 antibody to detect Aβ deposits (arrowheads). These deposits are larger and more numerous in the retromer-deficient flies. Each section shows a portion of the central neuropil located to the left and adjacent to the esophagus (Es). Anterior is toward the top. (c) Frontal brain sections at the midbrain level shown for control (+/+) and retromer-deficient (+/−) flies expressing human wild-type APP and human BACE. The left half of the central brain and optic lobe are shown. Esophagus is in the middle of the section at the right margin. Extensive neurodegeneration can be seen in the neuropil of retromer-deficient flies. (Scale bar: 50 μm.)
Similar articles
- Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.
Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ, Cai H, Kusnecov A, Cai Q. Ye X, et al. J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article. - Sphingosine Kinase 2 Potentiates Amyloid Deposition but Protects against Hippocampal Volume Loss and Demyelination in a Mouse Model of Alzheimer's Disease.
Lei M, Teo JD, Song H, McEwen HP, Yup Lee J, Couttas TA, Duncan T, Chesworth R, Bertz J, Przybyla M, Van Eersel J, Heng B, Guillemin GJ, Ittner LM, Fath T, Garner B, Ittner A, Karl T, Don AS. Lei M, et al. J Neurosci. 2019 Nov 27;39(48):9645-9659. doi: 10.1523/JNEUROSCI.0524-19.2019. Epub 2019 Oct 22. J Neurosci. 2019. PMID: 31641049 Free PMC article. - Familial Alzheimer's disease mutations at position 22 of the amyloid β-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system.
Tackenberg C, Kulic L, Nitsch RM. Tackenberg C, et al. PLoS One. 2020 Sep 23;15(9):e0239584. doi: 10.1371/journal.pone.0239584. eCollection 2020. PLoS One. 2020. PMID: 32966331 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - The Role of Retromer in Alzheimer's Disease.
Zhang QY, Tan MS, Yu JT, Tan L. Zhang QY, et al. Mol Neurobiol. 2016 Aug;53(6):4201-4209. doi: 10.1007/s12035-015-9366-0. Epub 2015 Jul 28. Mol Neurobiol. 2016. PMID: 26215837 Review.
Cited by
- VPS35 haploinsufficiency increases Alzheimer's disease neuropathology.
Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, Zhang QG, Brann D, Kim TW, Yan R, Mei L, Xiong WC. Wen L, et al. J Cell Biol. 2011 Nov 28;195(5):765-79. doi: 10.1083/jcb.201105109. Epub 2011 Nov 21. J Cell Biol. 2011. PMID: 22105352 Free PMC article. - Sorting receptors at Down's syndrome synapses.
Jones MW. Jones MW. Nat Med. 2013 Apr;19(4):404-6. doi: 10.1038/nm.3152. Nat Med. 2013. PMID: 23558625 No abstract available. - Novel crosstalk between Vps26a and Nox4 signaling during neurogenesis.
Choi SA, Kim YH, Park YH, Yang HJ, Jeong PS, Cha JJ, Yoon SB, Kim JS, Song BS, Lee JH, Sim BW, Huh JW, Song IS, Lee SR, Kim MK, Kim JM, Bae YS, Imakawa K, Kim SU, Chang KT. Choi SA, et al. Cell Death Differ. 2019 Sep;26(9):1582-1599. doi: 10.1038/s41418-018-0226-0. Epub 2018 Nov 21. Cell Death Differ. 2019. PMID: 30464227 Free PMC article. - Hyperleucinemia causes hippocampal retromer deficiency linking diabetes to Alzheimer's disease.
Morabito MV, Berman DE, Schneider RT, Zhang Y, Leibel RL, Small SA. Morabito MV, et al. Neurobiol Dis. 2014 May;65:188-92. doi: 10.1016/j.nbd.2013.12.017. Epub 2014 Jan 14. Neurobiol Dis. 2014. PMID: 24440570 Free PMC article. - Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5.
Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Seaman MN, et al. J Cell Sci. 2009 Jul 15;122(Pt 14):2371-82. doi: 10.1242/jcs.048686. Epub 2009 Jun 16. J Cell Sci. 2009. PMID: 19531583 Free PMC article.
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Scherzer CR, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–1205. - PubMed
- Small SA, et al. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann Neurol. 2005;58:909–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS015390/NS/NINDS NIH HHS/United States
- AG025161/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- R01 NS049442/NS/NINDS NIH HHS/United States
- NS48447/NS/NINDS NIH HHS/United States
- P01 NS048447/NS/NINDS NIH HHS/United States
- AG172116/AG/NIA NIH HHS/United States
- R01 AG025161/AG/NIA NIH HHS/United States
- NS049442/NS/NINDS NIH HHS/United States
- R01 NS015390/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases