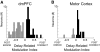Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex - PubMed (original) (raw)
Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex
Nandakumar S Narayanan et al. J Neurophysiol. 2008 Jul.
Abstract
Rats with impaired function in dorsomedial regions of the prefrontal cortex (dmPFC) are unable to maintain a behavioral response over a delay period. Here we report that neurons in this cortical region are prominently modulated after errors in a tone-cued, simple reaction time task and that inactivation of dmPFC attenuates a slowing of reaction times that is observed following errors. Using methods for chronic single-unit recording, we found that approximately one-third of dmPFC neurons were modulated after errors, and 28% of these neurons had increased posterror firing that persisted into the delay period of the following trial. In contrast to dmPFC, no such neurons were found in motor cortex. Our results implicate the dorsomedial prefrontal cortex in a form of retrospective working memory that improves task performance following errors.
Figures
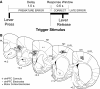
FIG. 1.
Behavioral task and electrode locations: Animals had to press and hold a lever for a 1.0-s delay period and release the lever promptly (i.e., within 0.6 s) to receive a liquid reward. The end of the delay period was signified by a 72-dB 8-kHz tone; in some recording sessions, the tone was omitted on 50% of trials. If animals released the lever prior to the end of the 1.0-s delay or after the 0.6-s response window, then these trials were scored as errors (premature or late, respectively), and all behavioral devices (pump, lever and houselight) were extinguished for 4–8 s. B: electrode locations: Recording sites in dorsomedial prefrontal cortex (dmPFC) are shown from 8 animals that were implanted with microwire electrodes arrays in dmPFC (white dots) and from 7 animals that were implanted with microwire electrode arrays in motor cortex (black dots) and cannula in dmPFC (black crosses). Reconstructions are shown in frontal planes based on atlas sections (Paxinos and Watson 1982).
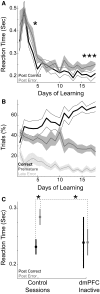
FIG. 2.
Posterror slowing of reaction times (RTs). A: RTs following correct trials (black line) decreased over learning but were consistently faster than RTs following error trials (gray line) only after the 16th day of training. Asterisk indicates significant posterror slowing (P < 0.05). B: learning of the simple RT task. Animals increased their correct responses (black line) over the course of several days, reaching 60% on the 11th day of training. Premature errors (dark gray line) decreased over training [paired T(1,6) = 10.2, P < 0.001 on day 1 vs. 11], and late errors (light gray line) decreased somewhat [paired T(1,6) = 2.00, P < 0.09 on day 1 vs. 11]. C: posterror slowing in control sessions was attenuated in sessions with dmPFC inactivated, as RTs became equivalent following errors and correct trials. Asterisk indicates significant posterror slowing (P < 0.05).
FIG. 3.
Errors influence persistent activity of dmPFC neurons. A: sequence of events following correct and error trials. Following correct trials (top line, black), a pump is activated at a latency of 100 ms after lever release and is kept on for 1 s. Following error trials (bottom line, gray) the house lights and all behavioral devices are extinguished for 4–8 s. Houselights then come on 1.5 ± 0.2 s prior to posterror lever presses. B–E: examples of neurons that fired differently depending on trial outcome are shown. B and C: neurons fired more if trials ended in error (gray colors, left panel) than if trials were correct and rewarded (black, left panel). Increased posterror firing (gray colors, right panel) persisted into the delay period of the following trial, and for these neurons, was more than postcorrect firing (black, right panel). Shaded region after lever press on right panel was used to identify delay-related posterror differences in firing rate. No difference was observed between premature (dark gray) and late (light gray) errors; see text.
FIG. 4.
Posterror modulation indices. Posterror (gray) or postcorrect (black) delay-related modulation indices plotted for dmPFC (A) and for motor cortex (B). Although dmPFC neurons could have greater firing rate following errors and correct trials, dmPFC had significantly more neurons with posterror delay activity than motor cortex, while motor cortex had significantly more neurons with postcorrect delay activity than dmPFC.
Similar articles
- Delay activity in rodent frontal cortex during a simple reaction time task.
Narayanan NS, Laubach M. Narayanan NS, et al. J Neurophysiol. 2009 Jun;101(6):2859-71. doi: 10.1152/jn.90615.2008. Epub 2009 Apr 1. J Neurophysiol. 2009. PMID: 19339463 Free PMC article. - Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex.
Narayanan NS, Laubach M. Narayanan NS, et al. Neuron. 2006 Dec 7;52(5):921-31. doi: 10.1016/j.neuron.2006.10.021. Neuron. 2006. PMID: 17145511 Free PMC article. - The role of rat dorsomedial prefrontal cortex in spatial working memory.
Horst NK, Laubach M. Horst NK, et al. Neuroscience. 2009 Dec 1;164(2):444-56. doi: 10.1016/j.neuroscience.2009.08.004. Epub 2009 Aug 7. Neuroscience. 2009. PMID: 19665526 Free PMC article. - Prefrontal cortex and working memory processes.
Funahashi S. Funahashi S. Neuroscience. 2006 Apr 28;139(1):251-61. doi: 10.1016/j.neuroscience.2005.07.003. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16325345 Review. - From perception to action: temporal integrative functions of prefrontal and parietal neurons.
Quintana J, Fuster JM. Quintana J, et al. Cereb Cortex. 1999 Apr-May;9(3):213-21. doi: 10.1093/cercor/9.3.213. Cereb Cortex. 1999. PMID: 10355901 Review.
Cited by
- Cortical neural responses to previous trial outcome during learning of a directional choice task.
Yuan Y, Mao H, Si J. Yuan Y, et al. J Neurophysiol. 2015 Apr 1;113(7):1963-76. doi: 10.1152/jn.00238.2014. Epub 2014 Dec 31. J Neurophysiol. 2015. PMID: 25552636 Free PMC article. - Anterior Cingulate Cortex Cells Identify Process-Specific Errors of Attentional Control Prior to Transient Prefrontal-Cingulate Inhibition.
Shen C, Ardid S, Kaping D, Westendorff S, Everling S, Womelsdorf T. Shen C, et al. Cereb Cortex. 2015 Aug;25(8):2213-28. doi: 10.1093/cercor/bhu028. Epub 2014 Mar 2. Cereb Cortex. 2015. PMID: 24591526 Free PMC article. - Adaptive Encoding of Outcome Prediction by Prefrontal Cortex Ensembles Supports Behavioral Flexibility.
Del Arco A, Park J, Wood J, Kim Y, Moghaddam B. Del Arco A, et al. J Neurosci. 2017 Aug 30;37(35):8363-8373. doi: 10.1523/JNEUROSCI.0450-17.2017. Epub 2017 Jul 20. J Neurosci. 2017. PMID: 28729442 Free PMC article. - Noradrenergic control of error perseveration in medial prefrontal cortex.
Caetano MS, Jin LE, Harenberg L, Stachenfeld KL, Arnsten AF, Laubach M. Caetano MS, et al. Front Integr Neurosci. 2013 Jan 2;6:125. doi: 10.3389/fnint.2012.00125. eCollection 2012. Front Integr Neurosci. 2013. PMID: 23293590 Free PMC article. - Neurochemical enhancement of conscious error awareness.
Hester R, Nandam LS, O'Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA. Hester R, et al. J Neurosci. 2012 Feb 22;32(8):2619-27. doi: 10.1523/JNEUROSCI.4052-11.2012. J Neurosci. 2012. PMID: 22357846 Free PMC article. Clinical Trial.
References
- Baddeley A Working Memory. Oxford, UK: Oxford Univ. Press, 1987.
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177–188, 2003. - PubMed
- Batuev AS, Kursina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res 41: 95–102, 1990. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources