A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats - PubMed (original) (raw)
. 2008 Jul 18;283(29):20361-71.
doi: 10.1074/jbc.M803225200. Epub 2008 May 15.
Greg Brown, Matthew D Zimmerman, Michael Proudfoot, Kira S Makarova, Marina Kudritska, Samvel Kochinyan, Shuren Wang, Maksymilian Chruszcz, Wladek Minor, Eugene V Koonin, Aled M Edwards, Alexei Savchenko, Alexander F Yakunin
Affiliations
- PMID: 18482976
- PMCID: PMC2459268
- DOI: 10.1074/jbc.M803225200
A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats
Natalia Beloglazova et al. J Biol Chem. 2008.
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPRs) together with the associated CAS proteins protect microbial cells from invasion by foreign genetic elements using presently unknown molecular mechanisms. All CRISPR systems contain proteins of the CAS2 family, suggesting that these uncharacterized proteins play a central role in this process. Here we show that the CAS2 proteins represent a novel family of endoribonucleases. Six purified CAS2 proteins from diverse organisms cleaved single-stranded RNAs preferentially within U-rich regions. A representative CAS2 enzyme, SSO1404 from Sulfolobus solfataricus, cleaved the phosphodiester linkage on the 3'-side and generated 5'-phosphate- and 3'-hydroxyl-terminated oligonucleotides. The crystal structure of SSO1404 was solved at 1.6A resolution revealing the first ribonuclease with a ferredoxin-like fold. Mutagenesis of SSO1404 identified six residues (Tyr-9, Asp-10, Arg-17, Arg-19, Arg-31, and Phe-37) that are important for enzymatic activity and suggested that Asp-10 might be the principal catalytic residue. Thus, CAS2 proteins are sequence-specific endoribonucleases, and we propose that their role in the CRISPR-mediated anti-phage defense might involve degradation of phage or cellular mRNAs.
Figures
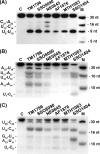
FIGURE 1.
Cleavage of ssRNAs by CAS2 proteins SSO1404, SSO8090, TM1796, NE0845, AF1876, and MTH1083. Autoradiograph of the 15% PAA, 8
m
urea gels showing the hydrolysis of RNA5 (A), RNA6 (B), and RNA14 (C) by the indicated CAS2 proteins. RNA substrates (0.1 μ
m
) were incubated at 37 °C for 45 min with the CAS2 protein (50 μg/ml) in the presence of 50 m
m
Tris-HCl (pH 8.5), 5 m
m
MgCl2, and 100 m
m
KCl. Reaction products were separated on a 15% PAAG, 8
m
urea gel as described under “Experimental Procedures.” Lane C represents the RNA sample incubated in the absence of protein; lane M shows the 5′-32P-labeled marker oligonucleotides.
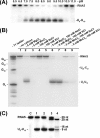
FIGURE 2.
Reaction requirements and RNA cleavage products of SSO1404. Autoradiographs of 15% PAA, 8
m
urea gels showing the effect of pH (A) or various metal cations (B) on the cleavage of RNA5 by SSO1404 (30 μg/ml). A, 5′-32P-labeled RNA5 (0.1 μ
m
) was incubated with SSO1404 at 37 °C for 10 min in a reaction mixture containing 50 m
m
buffer with the indicated pH (described under “Experimental Procedures”), 5 m
m
MgCl2, 100 m
m
KCl, and 1 m
m
dithiothreitol.B, 5′-32P-labeled RNA5 (0.5 μ
m
) was incubated with SSO1404 in the absence or presence of 5 m
m
MgCl2, 5 m
m
MnCl2, 100 m
m
NaCl, or 100 m
m
KCl. C, analysis of the RNA5 cleavage products generated by SSO1404: lane 1, reaction mixture without the addition of PNK; lane 2, reaction mixture treated with PNK (5′-end labeling reaction); lane 3, reaction mixture treated with PNK (5′-phosphate exchange reaction). Lane C represents the RNA sample incubated in the absence of protein; lane M shows the 5′-32P-labeled marker oligonucleotides. Reaction products were processed and visualized as described under “Experimental Procedures.” The position of the main RNA5 cleavage product_(U_9_-U_10) is shown on the_right_. A T1 ladder represents the products of RNA5 cleavage by RNase T1.
FIGURE 3.
RNase activity of SSO1404 with CRISPR-related substrates. A, cleavage of the 5′-fragment (270 nt) of the S. solfataricus CRISPR cluster-2 containing the cluster-2 upstream region, repeat-1, spacer-1, repeat-2, and a 27-nt fragment of spacer-2. Autoradiograph of the 8% PAA, 8
m
urea gel showing the cleavage of the uniformly labeled [32P]RNA (0.1 μ
m
) by SSO1404 (30 μg/ml). RNA was incubated in the absence of SSO1404 (30 min, lane 1) or in the presence of SSO1404 for (15 min, lane 2) or (30 min, lane 3). B, RNA1 to RNA5; C, RNA6 to RNA9; D, RNA10 to RNA12. The right part of the of B-D shows predicted secondary structures and the positions of the SSO1404 cleavage sites in selected RNA substrates. Im, imidazole.
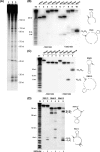
FIGURE 4.
Ribonuclease activity of SSO1404 against a series of the truncated RNA5 substrates. A, autoradiograph of the 15% PAA, 8
m
urea gel showing the activity of SSO1404 against the RNA5 substrate and its five truncated derivatives (the substrate lengths are shown on the right).B, sequences of the substrates used in the experiment and the positions of the cleavage sites of SSO1404. RNA substrates (0.1 μ
m
) were incubated for 15 min (37 °C) in the absence (lanes 1, 3, 5, 7, 9, and 11) or in the presence (lanes 2, 4, 6, 8, 10, and 12) of SSO1404 (30 μg/ml). Reaction products were processed and visualized as described under “Experimental Procedures.” Lanes T1 and Im represent the products of the RNA5 cleavage by RNase T1 and 2
m
imidazole (pH 7.0), respectively.
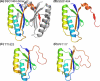
FIGURE 5.
Structures of SSO1404 and other CAS2 proteins. A, SSO1404 dimer. One of the two monomers is colored under “rainbow” representation (blue at the N terminus to red at the C terminus). B, SSO1404 monomer. C, TT1823 monomer (Protein Data Bank code 1zpw). D, PF1117 monomer (Protein Data Bank code 2i0x). All of the polypeptides are shown in equivalent orientations and are colored under rainbow representation.
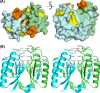
FIGURE 6.
Potential active site of SSO1404. A, surface representation (two orientations) of the SSO1404 dimer showing the potential active site: the deep cavity between two monomers harboring two conserved Asp-10 (colored_red_). One of the monomers is colored in cyan, and the other is in green. Residues colored orange (Gln-63, Glu-64, and Asp-65) are located on the two α2-β4 loops, which are predicted to be involved in RNA binding. Other conserved residues important for activity are shown in yellow (Tyr-9, Arg-17, Arg-19, Arg-31, and Phe-37).B, stereo view of the SSO1404 dimer showing the positions of the residues important for activity (Tyr-9, Asp-10, Arg-17, Arg-19, Arg-31, and Phe-37). One of the monomers is colored in cyan, and the other is in_green_. The orientation of the SSO1404 dimer in B corresponds to the right model of A. Note the proximity of the two principal catalytic Asp-10 residues in the dimer (6.5 Å).
FIGURE 7.
Structure-based sequence alignment of SSO1404 and selected CAS2 proteins. Residues conserved in all CAS2 proteins are highlighted in_black_, highly conserved residues in dark gray, and similar residues in light gray. The secondary structure elements derived from the structure of SSO1404 (Protein Data Bank code 2i8e) are shown above the alignment. The asterisks designate the residues important for the catalytic activity of SSO1404. The compared proteins are as follows:SSO1404(Q97YC2), SSO8090(Q97Y85), TT1823 from T. thermophilus(Q746F4), PF1117 from P. furiosus (Q8U1T8), AF1876 from A. fulgidus (O28403), MTH1083 from M. thermoautotrophicum (O27155), TM1796 from T. maritima (Q9X2B6), and NE0845 from N. europaea (Q82W51). The sequences were aligned using both sequence and structural information by 3DCOFFEE (63), and the figure was generated using TEXSHADE (64).
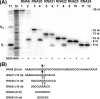
FIGURE 8.
Site-directed mutagenesis of SSO1404, RNase activity of purified mutant proteins with the RNA5 substrate. Autoradiograph of the 15% PAA, 8
m
urea gel showing the cleavage of 5-32P-labeled RNA5 (0.1 μ
m
) by purified proteins (30 μg/ml). Reaction mixtures were incubated at 37 °C for 10 min, and the reaction products were processed as described under “Experimental Procedures.” Lane C represents the RNA5 sample incubated without protein addition.
Similar articles
- Conservation and variability in the structure and function of the Cas5d endoribonuclease in the CRISPR-mediated microbial immune system.
Koo Y, Ka D, Kim EJ, Suh N, Bae E. Koo Y, et al. J Mol Biol. 2013 Oct 23;425(20):3799-810. doi: 10.1016/j.jmb.2013.02.032. Epub 2013 Mar 7. J Mol Biol. 2013. PMID: 23500492 - Recognition and cleavage of a nonstructured CRISPR RNA by its processing endoribonuclease Cas6.
Shao Y, Li H. Shao Y, et al. Structure. 2013 Mar 5;21(3):385-93. doi: 10.1016/j.str.2013.01.010. Structure. 2013. PMID: 23454186 Free PMC article. - Sequence- and structure-specific RNA processing by a CRISPR endonuclease.
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Haurwitz RE, et al. Science. 2010 Sep 10;329(5997):1355-8. doi: 10.1126/science.1192272. Science. 2010. PMID: 20829488 Free PMC article. - Cutting it close: CRISPR-associated endoribonuclease structure and function.
Hochstrasser ML, Doudna JA. Hochstrasser ML, et al. Trends Biochem Sci. 2015 Jan;40(1):58-66. doi: 10.1016/j.tibs.2014.10.007. Epub 2014 Nov 18. Trends Biochem Sci. 2015. PMID: 25468820 Review. - Cas6 processes tight and relaxed repeat RNA via multiple mechanisms: A hypothesis.
Sefcikova J, Roth M, Yu G, Li H. Sefcikova J, et al. Bioessays. 2017 Jun;39(6):10.1002/bies.201700019. doi: 10.1002/bies.201700019. Epub 2017 May 11. Bioessays. 2017. PMID: 28493337 Free PMC article. Review.
Cited by
- CRISPR-Cas systems target endogenous genes to impact bacterial physiology and alter mammalian immune responses.
Wu Q, Cui L, Liu Y, Li R, Dai M, Xia Z, Wu M. Wu Q, et al. Mol Biomed. 2022 Jul 20;3(1):22. doi: 10.1186/s43556-022-00084-1. Mol Biomed. 2022. PMID: 35854035 Free PMC article. Review. - Adapting to new threats: the generation of memory by CRISPR-Cas immune systems.
Heler R, Marraffini LA, Bikard D. Heler R, et al. Mol Microbiol. 2014 Jul;93(1):1-9. doi: 10.1111/mmi.12640. Epub 2014 Jun 4. Mol Microbiol. 2014. PMID: 24806524 Free PMC article. Review. - Pdxdc1 modulates prepulse inhibition of acoustic startle in the mouse.
Feldcamp LA, Boutros PC, Raymond R, Fletcher PJ, Nobrega JN, Wong AHC. Feldcamp LA, et al. Transl Psychiatry. 2017 May 9;7(5):e1125. doi: 10.1038/tp.2017.85. Transl Psychiatry. 2017. PMID: 28485732 Free PMC article. - RNA damage in biological conflicts and the diversity of responding RNA repair systems.
Burroughs AM, Aravind L. Burroughs AM, et al. Nucleic Acids Res. 2016 Oct 14;44(18):8525-8555. doi: 10.1093/nar/gkw722. Epub 2016 Aug 17. Nucleic Acids Res. 2016. PMID: 27536007 Free PMC article. Review. - In vivo protein interactions and complex formation in the Pectobacterium atrosepticum subtype I-F CRISPR/Cas System.
Richter C, Gristwood T, Clulow JS, Fineran PC. Richter C, et al. PLoS One. 2012;7(12):e49549. doi: 10.1371/journal.pone.0049549. Epub 2012 Dec 3. PLoS One. 2012. PMID: 23226499 Free PMC article.
References
- Jansen, R., Embden, J. D., Gaastra, W., and Schouls, L. M. (2002) Mol. Microbiol. 43 1565-1575 - PubMed
- Mojica, F. J., Diez-Villasenor, C., Soria, E., and Juez, G. (2000) Mol. Microbiol. 36 244-246 - PubMed
- Sorek, R., Kunin, V., and Hugenholtz, P. (2008) Nat. Rev. Microbiol. 6 181-186 - PubMed
- Groenen, P. M., Bunschoten, A. E., van Soolingen, D., and van Embden, J. D. (1993) Mol. Microbiol. 10 1057-1065 - PubMed
- Mojica, F. J., Diez-Villasenor, C., Garcia-Martinez, J., and Soria, E. (2005) J. Mol. Evol. 60 174-182 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases