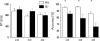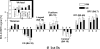Impairment of attentional networks after 1 night of sleep deprivation - PubMed (original) (raw)
Impairment of attentional networks after 1 night of sleep deprivation
D Tomasi et al. Cereb Cortex. 2009 Jan.
Abstract
Here, we assessed the effects of sleep deprivation (SD) on brain activation and performance to a parametric visual attention task. Fourteen healthy subjects underwent functional magnetic resonance imaging of ball-tracking tasks with graded levels of difficulty during rested wakefulness (RW) and after 1 night of SD. Self-reports of sleepiness were significantly higher and cognitive performance significantly lower for all levels of difficulty for SD than for RW. For both the RW and the SD sessions, task difficulty was associated with activation in parietal cortex and with deactivation in visual and insular cortices and cingulate gyrus but this pattern of activation/deactivation was significantly lower for SD than for RW. In addition, thalamic activation was higher for SD than for RW, and task difficulty was associated with increases in thalamic activation for the RW but not the SD condition. This suggests that thalamic resources, which under RW conditions are used to process increasingly complex tasks, are being used to maintain alertness with increasing levels of fatigue during SD. Thalamic activation was also inversely correlated with parietal and prefrontal activation. Thus, the thalamic hyperactivation during SD could underlie the reduced activation in parietal and blunted deactivation in cingulate cortices, impairing the attentional networks that are essential for accurate visuospatial attention performance.
Figures
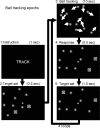
Figure 1.
Outline of TRACK epochs of the 2-ball–tracking task. Subjects track the target ball set, which is briefly highlighted (frame 2) after the instruction (frame 1), while all 10 balls move with a random motion for 10 s (frame 3). Then, they respond with a button press if the highlighted balls are those they were tracking (frame 4). The target set if rehighlighted to refocus the subjects’ attention on the balls (frame 5).
Figure 2.
Performance accuracy and RTs for the RW (white) and SD (black) sessions, as a function of the number of tracked balls (VA load). Sample size: 14 healthy men.
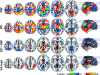
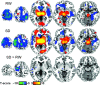
Figure 3.
BOLD fMRI activation patterns for the main (RW, SD) and differential (SD > RW and VA load: 4 balls > 2 balls) effects of visual attention, rendered to a structural MRI image. Random-effects analyses (1-way within-subjects ANOVA).
Figure 4.
Average BOLD fMRI signals in specific ROIs (Table 1). Volume = 1.46 cc (left and right ROIs averaged). Sample size: 14 healthy nonsmoking men.
Figure 5.
Statistical maps of functional thalamocortical connectivity during resting epochs, across all 14 healthy subjects and ball-tracking conditions (2, 3, and 4 balls), superimposed to axial slices of a reference brain. Top row: conjunctive analysis of the RW and SD sessions; middle and bottom rows analyses of individual sessions. SPM2 random-effects model: within-subjects ANOVA.
Similar articles
- Functional imaging of working memory after 24 hr of total sleep deprivation.
Chee MW, Choo WC. Chee MW, et al. J Neurosci. 2004 May 12;24(19):4560-7. doi: 10.1523/JNEUROSCI.0007-04.2004. J Neurosci. 2004. PMID: 15140927 Free PMC article. - Sleep deprivation impairs object-selective attention: a view from the ventral visual cortex.
Lim J, Tan JC, Parimal S, Dinges DF, Chee MW. Lim J, et al. PLoS One. 2010 Feb 5;5(2):e9087. doi: 10.1371/journal.pone.0009087. PLoS One. 2010. PMID: 20140099 Free PMC article. Clinical Trial. - How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies.
Ma N, Dinges DF, Basner M, Rao H. Ma N, et al. Sleep. 2015 Feb 1;38(2):233-40. doi: 10.5665/sleep.4404. Sleep. 2015. PMID: 25409102 Free PMC article. - Functional imaging of brain responses to pain. A review and meta-analysis (2000).
Peyron R, Laurent B, García-Larrea L. Peyron R, et al. Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review. - The effects of total sleep deprivation on cerebral responses to cognitive performance.
Drummond SP, Brown GG. Drummond SP, et al. Neuropsychopharmacology. 2001 Nov;25(5 Suppl):S68-73. doi: 10.1016/S0893-133X(01)00325-6. Neuropsychopharmacology. 2001. PMID: 11682277 Review.
Cited by
- Effects of sleep deprivation on central auditory processing.
Liberalesso PB, D'Andrea KF, Cordeiro ML, Zeigelboim BS, Marques JM, Jurkiewicz AL. Liberalesso PB, et al. BMC Neurosci. 2012 Jul 23;13:83. doi: 10.1186/1471-2202-13-83. BMC Neurosci. 2012. PMID: 22823997 Free PMC article. - Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment.
Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Wang RL, Logan J, Wong C, Jayne M, Swanson JM. Volkow ND, et al. Neuroimage. 2009 May 1;45(4):1232-40. doi: 10.1016/j.neuroimage.2009.01.003. Epub 2009 Jan 20. Neuroimage. 2009. PMID: 19349237 Free PMC article. - Age effect on gray matter volume changes after sleep restriction.
Long Z, Cheng F, Lei X. Long Z, et al. PLoS One. 2020 Feb 6;15(2):e0228473. doi: 10.1371/journal.pone.0228473. eCollection 2020. PLoS One. 2020. PMID: 32027695 Free PMC article. Clinical Trial. - Altered Regional Homogeneity in Chronic Insomnia Disorder with or without Cognitive Impairment.
Pang R, Guo R, Wu X, Hu F, Liu M, Zhang L, Wang Z, Li K. Pang R, et al. AJNR Am J Neuroradiol. 2018 Apr;39(4):742-747. doi: 10.3174/ajnr.A5587. Epub 2018 Mar 1. AJNR Am J Neuroradiol. 2018. PMID: 29496724 Free PMC article. - Disrupted directed connectivity along the cingulate cortex determines vigilance after sleep deprivation.
Piantoni G, Cheung BL, Van Veen BD, Romeijn N, Riedner BA, Tononi G, Van Der Werf YD, Van Someren EJ. Piantoni G, et al. Neuroimage. 2013 Oct 1;79:213-22. doi: 10.1016/j.neuroimage.2013.04.103. Epub 2013 May 3. Neuroimage. 2013. PMID: 23643925 Free PMC article.
References
- Adler C, Sax K, Holland S, Schmithorst V, Rosenberg L, Strakowski S. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. - PubMed
- Akert K, Monakow K, Künzle H. Projection of precentral motor cortex upon nucleus medialis dorsalis thalami in the monkey. Neurosci Lett. 1979;11:103–106. - PubMed
- Arrington C, Carr T, Mayer A, Rao S. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12(Suppl 2):106–117. - PubMed
- Ashburner J, Neelin P, Collins DL, Evans AC, Friston KJ. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–352. - PubMed