Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation - PubMed (original) (raw)
Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation
Jutong Si et al. Cancer Res. 2008.
Abstract
Ca(2+) signaling is an important component of signal transduction pathways regulating B and T lymphocyte proliferation, but the functional role of Ca(2+) signaling in regulating myeloid leukemia cell proliferation has been largely unexplored. We observe that the activated (autophosphorylated) Ca(2+)/calmodulin-dependent protein kinase IIgamma (CaMKIIgamma) is invariably present in myeloid leukemia cell lines as well as in the majority of primary acute myelogenous leukemia patient samples. In contrast, myeloid leukemia cells induced to terminally differentiate or undergo growth arrest display a marked reduction in this CaMKIIgamma autophosphorylation. In cells harboring the bcr-abl oncogene, the activation (autophosphorylation) of CaMKIIgamma is regulated by this oncogene. Moreover, inhibition of CaMKIIgamma activity with pharmacologic agents, dominant-negative constructs, or short hairpin RNAs inhibits the proliferation of myeloid leukemia cells, and this is associated with the inactivation/down-regulation of multiple critical signal transduction networks involving the mitogen-activated protein kinase, Janus-activated kinase/signal transducers and activators of transcription (Jak/Stat), and glycogen synthase kinase (GSK3beta)/beta-catenin pathways. In myeloid leukemia cells, CaMKIIgamma directly phosphorylates Stat3 and enhances its transcriptional activity. Thus, CaMKIIgamma is a critical regulator of multiple signaling networks regulating the proliferation of myeloid leukemia cells. Inhibiting CaMKIIgamma may represent a novel approach in the targeted therapy of myeloid leukemia.
Figures
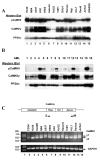
Figure 1. CaMKIIγ activation in myeloid leukemia cells
A). Cell lysates from the indicated human leukemia cell lines were subjected to Western blots utilizing the indicated CaMKII antibodies. Antibodies to the catalytic subunit of PP2A (PP2Ac) serve as a loading control. B). Cell lysates from primary AML samples were subjected to Western blots utilizing the indicated CaMKII antibodies. Antibodies to PP2Ac serve as a loading control. C) RT-PCR was performed on RNA extracted from the indicated leukemia cell lines and normal CD34+ cells and the products were displayed on an agarose gel. The location of the sense (S) and antisense (AS) primers in relationship to the catalytic, regulatory, variable and association domains of the human CaMKIIγ coding sequence are indicated.
Figure 2. Terminal differentiation/apoptosis of myeloid leukemia cells is associated with decreased CaMKIIγ activation
A). Lysates from the HL60, NB4 and U937 myeloid cell lines treated with ATRA (1μM) for the indicated period of time were subjected to Western blots. B). Lysates from the indicated myeloid cell lines treated for five days with the indicated concentration of ATRA were subjected to Western blots. C). Lysates from HL60 cells treated with TPA (0.1 μM) for the indicated time were subjected to Western blots. D). Lysates from NB4 cells treated with arsenic trioxide (1 uM) for the indicated time were subjected to Western blots.
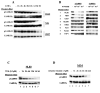
Figure 3. Inhibiting bcr-abl activity/expression inhibits CaMKIIγ activation
A). Lysates from K562 cells undergoing growth arrest following treatment with gleevec (5uM) for the indicated times were subjected to Western blots. B). Lysates from TonB210.1 cells depleted of doxycycline for the indicated period of time were subjected to Western blots. C). IL-3 dependent TonB210.1 cells were deprived of IL-3 for 24 hours (lane 1). The cells were then treated with the indicated compounds simultaneously with the addition of doxycycline (lanes 2-8). After 24 hours cell lysates were harvested and subjected to Western blots with the indicated CaMKII antibodies. D). K562 cells were cultured for 48 hours with the indicated chemical inhibitors of specific signal transduction pathways. Cell lysates were harvested and subjected to Western blot analysis.
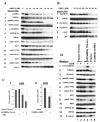
Figure 4. CaMKII inhibitors inhibit myeloid leukemia cell proliferation
A). K562 cells were seeded in liquid suspension culture at 5 × 104 cells/ml in the presence or absence of the indicated concentration of compounds, and cell counts were obtained at the indicated time. B). The indicated myeloid leukemia cell lines were seeded in liquid suspension culture at 5 × 104 cells/ml in the presence or absence of KN93, and cell counts obtained at the indicated time. C). K562 cells were electroporated with the LXSN expression vector harboring the kinase dead, Lys43 mutated, truncated CaMKII construct (kdCaMKII) as well as with the control (empty) LXSN vector. The electroporated cells were diluted into 96 well plates in liquid suspension culture in the presence of G418 (1 mg/ml). Following eight days of culture the total number of discrete, actively proliferating G418 resistant colonies within individual wells was determined.
Figure 5. CaMKII regulates multiple signal transduction pathways in myeloid cells
(A,B). Lysates from K562 cells cultured for the indicated time in KN93 were subjected to Western blots. (C) The K562 cells transduced as described in Materials and Methods with either (i) the doxycycline (DOX) inducible expression vector/transactivator harboring the full length, Lys43 mutated, kinase dead CaMKIIγ (kdCaMKIIγ (Tet-on)) or (ii) the CaMKII shRNA generating plasmids, were cultured in liquid suspension in the presence or absence of DOX as indicated, and cell counts obtained after 48 hours. (D) Lysates from K562 cells transduced with the indicated vectors were subjected to Western blots utilizing the indicated antibodies. For lanes 2 and 4 doxycycline (DOX) treatment was for two days.
Figure 6. Stat3 is directly phosphorylated and activated by CaMKIIγ at Ser727
A, i). Cell lysates from the indicated human leukemia cell lines were subjected to Western blots utilizing the indicated Stat3 antibodies. ii). Cell lysates from the same primary AML samples depicted in Fig. 1B were subjected to Western blots utilizing the indicated Stat3 antibodies. B). The IL-3 dependent TonB210.1 cells were deprived of IL-3 for 24 hours. The cells were then treated with the indicated compounds immediately prior to the addition of doxycycline. After an additional 24 hours cell lysates were harvested and subjected to Western blots with the indicated Stat3 antibodies. C). K562 cells were electroporated with a luciferase reporter driven by a Stat3 response element. The indicated concentrations of KN93 and the Stat 3 inhibitor, cucurbitacin I, were added and relative luciferase activity determined on cell lysates following 48 hours of culture. D, i) K562 cell lysates were immunoprecipitated with control IgG or a Stat3 antibody. The immunoprecipitates were then subjected to Western blot analysis with the indicated antibodies. ii, ) A bacterial - expressed GST-Stat3 fusion protein was incubated in vitro with CaMKIIγ that had been immunoprecipitated from HL60 cells. Western blots were then performed on the reaction mixtures utilizing the indicated Stat3 antibodies. iii,). NIH3T3 cells were transfected with the empty LXSN expression vector (lane 1), the same vector harboring the coding sequences of a constitutively active (ca) CaMKIIγ (lane 2), or vector harboring the kinase dead (kd) CaMKIIγ (lane 3). After 48 hours Western blots were performed on cell lysates utilizing the indicated Stat3 antibodies.
References
- Gilliland DG. Molecular genetics of human leukemias: new insights into therapy. Semin Hematol. 2002;39:6–11. - PubMed
- Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–215. - PubMed
- Pabst T, Mueller BU, Zhang P, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–70. - PubMed
- Beghini A, Ripamonti CB, Cairoli R, et al. KIT activating mutations: incidence in adult and pediatric acute myeloid leukemia, and identification of an internal tandem duplication. Haematologica. 2004;89:920–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous