Spontaneous access to DNA target sites in folded chromatin fibers - PubMed (original) (raw)
Spontaneous access to DNA target sites in folded chromatin fibers
Michael G Poirier et al. J Mol Biol. 2008.
Abstract
DNA wrapped in nucleosomes is sterically occluded from many protein complexes that must act on it; how such complexes gain access to nucleosomal DNA is not known. In vitro studies on isolated nucleosomes show that they undergo spontaneous partial unwrapping conformational transitions, which make the wrapped nucleosomal DNA transiently accessible. Thus, site exposure might provide a general mechanism allowing access of protein complexes to nucleosomal DNA. However, existing quantitative analyses of site exposure focused on single nucleosomes, while the presence of neighbor nucleosomes and concomitant chromatin folding might significantly influence site exposure. In this work, we carried out quantitative studies on the accessibility of nucleosomal DNA in homogeneous nucleosome arrays. Two striking findings emerged. Organization into chromatin fibers changes the accessibility of nucleosomal DNA only modestly, from approximately 3-fold decreases to approximately 8-fold increases in accessibility. This means that nucleosome arrays are intrinsically dynamic and accessible even when they are visibly condensed. In contrast, chromatin folding decreases the accessibility of linker DNA by as much as approximately 50-fold. Thus, nucleosome positioning dramatically influences the accessibility of target sites located inside nucleosomes, while chromatin folding dramatically regulates access to target sites in linker DNA.
Figures
Figure 1
Restriction enzyme digestion assay for site exposure. k12 and k21 are the forward and reverse rates of site exposure of a unique DNA site in a single nucleosome; k12* and k21* are the corresponding rates for exposure of a unique site in a nucleosome array; k23 and k32 are the rates for restriction enzyme binding and unbinding; and k4 is the rate of restriction enzyme catalysis. The restriction enzyme kinetics method determines the equilibrium constant for site exposure, Kequ = k12 / k21 or = k12* / k21*.
Figure 2
DNA templates used. The nucleosome positioning sequence mp1 is a variant of positioning sequence 601, while mp2 is a variant of 601.2. mp2 contains many restriction enzyme recognition sites that are not present in mp1, allowing analysis of accessibility at unique sites within the dimer and 17-mer nucleosome arrays.
Figure 3
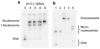
Reconstitution, purification, and characterization of mono- and di-nucleosomes. (a) Native 5% polyacrylamide gel of dinucleosome template DNA reconstituted with increasing concentrations of histone octamer. The shifts in DNA mobility are due to the formation of one and then two nucleosomes on each DNA. Numbers at the top (0–0.8) indicate the mass ratio (w/w) of histone octamer to total DNA used in the reconstitution reaction in that lane. Total DNA includes both the specific (high affinity) dinucleosome template DNA plus low affinity cpDNA competitor present as a histone buffer (see Methods). The specific template DNA saturates with histone octamer even though the amount of octamer is substoichiometric for the total amount of DNA. (b) Mononucleosome (lanes 1, 2) and dinucleosome (lanes 3, 4) reconstitutions before (lanes 1, 3) and after (lanes 2, 4) purification on sucrose gradients. Lane 5, purified dinucleosomes after digestion with 20 units ml−1 Sac I for 1 hour. Sac I cuts in the linker region, resulting in two bands having gel mobilities similar to that of mononucleosomes (lane 2), and two faint bands from digested naked template DNA (e.g., any template DNA that was not incorporated into nucleosomes). Quantification of these bands shows that greater than 95% of the dinucleosome’s positioning sequences are wrapped into nucleosomes.
Figure 4
Reconstitution, purification, and characterization of nucleosome 17-mers. (a) Native gel analysis of 17-mer template DNA reconstituted with increasing concentrations of histone octamer. The mass ratio (w/w) of histone octamer to total DNA used in the reconstitution reaction in each lane is indicated. The decrease in mobility is due to nucleosome formation along the DNA template. The mass ratios 0.1 and 0.2 have wide range of gel mobilities owing to the inherent heterogeneity of DNA templates that are partially filled with nucleosomes. In contrast, the mass ratios of 0.4 and 0.8 yield a well-defined shift in gel mobility, suggesting that the DNA template is saturated with nucleosomes. (b) Ethidium-stained native gel analysis of the 17-mer DNA template (lane 1), and reconstituted nucleosome array (0.8 histone to DNA mass ratio) before (lane 2) and after purification (lane 3). The sucrose gradient removes the short low affinity competitor DNA and any aggregates. (c) Native 5% polyacrylamide gel analysis of DNA products from a Mlu I digestion of purified 17-mer nucleosomes mixed with naked DNA. The 17-mer contains 16 Mlu I sites and the naked DNA contains a single MluI site. (d) Quantification of results from (c) showing the fraction of naked DNA and the nucleosome 17-mer remaining uncut, as a function of time. The curves show best fits to the appropriate exponential decays (see Methods). These results show that 0.03 of the positioning sequences are not incorporated into nucleosomes, i.e., that 97 % of all positioning sequences in these nucleosome 17-mers are wrapped into nucleosomes.
Figure 4
Reconstitution, purification, and characterization of nucleosome 17-mers. (a) Native gel analysis of 17-mer template DNA reconstituted with increasing concentrations of histone octamer. The mass ratio (w/w) of histone octamer to total DNA used in the reconstitution reaction in each lane is indicated. The decrease in mobility is due to nucleosome formation along the DNA template. The mass ratios 0.1 and 0.2 have wide range of gel mobilities owing to the inherent heterogeneity of DNA templates that are partially filled with nucleosomes. In contrast, the mass ratios of 0.4 and 0.8 yield a well-defined shift in gel mobility, suggesting that the DNA template is saturated with nucleosomes. (b) Ethidium-stained native gel analysis of the 17-mer DNA template (lane 1), and reconstituted nucleosome array (0.8 histone to DNA mass ratio) before (lane 2) and after purification (lane 3). The sucrose gradient removes the short low affinity competitor DNA and any aggregates. (c) Native 5% polyacrylamide gel analysis of DNA products from a Mlu I digestion of purified 17-mer nucleosomes mixed with naked DNA. The 17-mer contains 16 Mlu I sites and the naked DNA contains a single MluI site. (d) Quantification of results from (c) showing the fraction of naked DNA and the nucleosome 17-mer remaining uncut, as a function of time. The curves show best fits to the appropriate exponential decays (see Methods). These results show that 0.03 of the positioning sequences are not incorporated into nucleosomes, i.e., that 97 % of all positioning sequences in these nucleosome 17-mers are wrapped into nucleosomes.
Figure 4
Reconstitution, purification, and characterization of nucleosome 17-mers. (a) Native gel analysis of 17-mer template DNA reconstituted with increasing concentrations of histone octamer. The mass ratio (w/w) of histone octamer to total DNA used in the reconstitution reaction in each lane is indicated. The decrease in mobility is due to nucleosome formation along the DNA template. The mass ratios 0.1 and 0.2 have wide range of gel mobilities owing to the inherent heterogeneity of DNA templates that are partially filled with nucleosomes. In contrast, the mass ratios of 0.4 and 0.8 yield a well-defined shift in gel mobility, suggesting that the DNA template is saturated with nucleosomes. (b) Ethidium-stained native gel analysis of the 17-mer DNA template (lane 1), and reconstituted nucleosome array (0.8 histone to DNA mass ratio) before (lane 2) and after purification (lane 3). The sucrose gradient removes the short low affinity competitor DNA and any aggregates. (c) Native 5% polyacrylamide gel analysis of DNA products from a Mlu I digestion of purified 17-mer nucleosomes mixed with naked DNA. The 17-mer contains 16 Mlu I sites and the naked DNA contains a single MluI site. (d) Quantification of results from (c) showing the fraction of naked DNA and the nucleosome 17-mer remaining uncut, as a function of time. The curves show best fits to the appropriate exponential decays (see Methods). These results show that 0.03 of the positioning sequences are not incorporated into nucleosomes, i.e., that 97 % of all positioning sequences in these nucleosome 17-mers are wrapped into nucleosomes.
Figure 5
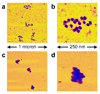
Atomic Force Microscopy images of nucleosome 17-mers. (a, b) purified nucleosome 17-mers adsorbed onto mica in 0.2 × TE (which causes them to adopt extended conformations) and imaged in air; (b) 4× zoom of the upper array in (a). These and additional images confirm the biochemical results showing that the 17-mers are greater than 95% saturated with nucleosomes. (c, d) 17-mers adsorbed in 0.5× TE plus 1 mM MgCl2 (which allows them to compact) and imaged in that buffer. (d) is a 4× zoom of the lower array in (c). As expected, the arrays fold into more compact structures in the presence of MgCl2.
Figure 6
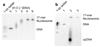
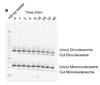
Site exposure in dinucleosomes and nucleosome 17-mers measured relative to mononucleosomes. (a) Native gel analysis of DNA products from Hae III digestions of a mixture of purified dinucleosomes and mononucleosomes; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay (see Methods). (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosomes and nucleosome 17-mers, measured relative to accessibilities in mononucleosomes, for sites spanning the test nucleosome. Averages and standard deviations (n=2 or 3) are shown.
Figure 6
Site exposure in dinucleosomes and nucleosome 17-mers measured relative to mononucleosomes. (a) Native gel analysis of DNA products from Hae III digestions of a mixture of purified dinucleosomes and mononucleosomes; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay (see Methods). (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosomes and nucleosome 17-mers, measured relative to accessibilities in mononucleosomes, for sites spanning the test nucleosome. Averages and standard deviations (n=2 or 3) are shown.
Figure 6
Site exposure in dinucleosomes and nucleosome 17-mers measured relative to mononucleosomes. (a) Native gel analysis of DNA products from Hae III digestions of a mixture of purified dinucleosomes and mononucleosomes; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay (see Methods). (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosomes and nucleosome 17-mers, measured relative to accessibilities in mononucleosomes, for sites spanning the test nucleosome. Averages and standard deviations (n=2 or 3) are shown.
Figure 6
Site exposure in dinucleosomes and nucleosome 17-mers measured relative to mononucleosomes. (a) Native gel analysis of DNA products from Hae III digestions of a mixture of purified dinucleosomes and mononucleosomes; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay (see Methods). (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosomes and nucleosome 17-mers, measured relative to accessibilities in mononucleosomes, for sites spanning the test nucleosome. Averages and standard deviations (n=2 or 3) are shown.
Figure 6
Site exposure in dinucleosomes and nucleosome 17-mers measured relative to mononucleosomes. (a) Native gel analysis of DNA products from Hae III digestions of a mixture of purified dinucleosomes and mononucleosomes; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay (see Methods). (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosomes and nucleosome 17-mers, measured relative to accessibilities in mononucleosomes, for sites spanning the test nucleosome. Averages and standard deviations (n=2 or 3) are shown.
Figure 7
Site exposure in linker DNA within dinucleosomes and nucleosome 17-mers measured relative to naked DNA. (a) Native gel analysis of DNA products from Sac I digestions of a mixture of purified dinucleosomes and naked DNA; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay. (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosome and nucleosome 17-mer linker DNA, measured relative to accessibilities in naked DNA, for sites spanning one linker DNA region. Averages and standard deviations (n=2–5) are shown.
Figure 7
Site exposure in linker DNA within dinucleosomes and nucleosome 17-mers measured relative to naked DNA. (a) Native gel analysis of DNA products from Sac I digestions of a mixture of purified dinucleosomes and naked DNA; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay. (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosome and nucleosome 17-mer linker DNA, measured relative to accessibilities in naked DNA, for sites spanning one linker DNA region. Averages and standard deviations (n=2–5) are shown.
Figure 7
Site exposure in linker DNA within dinucleosomes and nucleosome 17-mers measured relative to naked DNA. (a) Native gel analysis of DNA products from Sac I digestions of a mixture of purified dinucleosomes and naked DNA; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay. (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosome and nucleosome 17-mer linker DNA, measured relative to accessibilities in naked DNA, for sites spanning one linker DNA region. Averages and standard deviations (n=2–5) are shown.
Figure 7
Site exposure in linker DNA within dinucleosomes and nucleosome 17-mers measured relative to naked DNA. (a) Native gel analysis of DNA products from Sac I digestions of a mixture of purified dinucleosomes and naked DNA; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay. (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosome and nucleosome 17-mer linker DNA, measured relative to accessibilities in naked DNA, for sites spanning one linker DNA region. Averages and standard deviations (n=2–5) are shown.
Figure 7
Site exposure in linker DNA within dinucleosomes and nucleosome 17-mers measured relative to naked DNA. (a) Native gel analysis of DNA products from Sac I digestions of a mixture of purified dinucleosomes and naked DNA; times of digestion (minutes) are indicated. (b) Quantification of the fraction of uncut DNA from (a), fit with an exponential decay. (c, d) As in (a, b) except purified nucleosome 17-mers are used in place of dinucleosomes. (e) Summary of site accessibilities in dinucleosome and nucleosome 17-mer linker DNA, measured relative to accessibilities in naked DNA, for sites spanning one linker DNA region. Averages and standard deviations (n=2–5) are shown.
Similar articles
- Rapid spontaneous accessibility of nucleosomal DNA.
Li G, Levitus M, Bustamante C, Widom J. Li G, et al. Nat Struct Mol Biol. 2005 Jan;12(1):46-53. doi: 10.1038/nsmb869. Epub 2004 Dec 5. Nat Struct Mol Biol. 2005. PMID: 15580276 - HMG-D and histone H1 alter the local accessibility of nucleosomal DNA.
Ragab A, Travers A. Ragab A, et al. Nucleic Acids Res. 2003 Dec 15;31(24):7083-9. doi: 10.1093/nar/gkg923. Nucleic Acids Res. 2003. PMID: 14654683 Free PMC article. - Characterization of nucleosome unwrapping within chromatin fibers using magnetic tweezers.
Chien FT, van der Heijden T. Chien FT, et al. Biophys J. 2014 Jul 15;107(2):373-383. doi: 10.1016/j.bpj.2014.05.036. Biophys J. 2014. PMID: 25028879 Free PMC article. - The nucleosomal array: structure/function relationships.
Fletcher TM, Hansen JC. Fletcher TM, et al. Crit Rev Eukaryot Gene Expr. 1996;6(2-3):149-88. doi: 10.1615/critreveukargeneexpr.v6.i2-3.40. Crit Rev Eukaryot Gene Expr. 1996. PMID: 8855387 Review. - Chromatin structures condensed by linker histones.
Zhou BR, Bai Y. Zhou BR, et al. Essays Biochem. 2019 Apr 23;63(1):75-87. doi: 10.1042/EBC20180056. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 31015384 Review.
Cited by
- Nucleosomes undergo slow spontaneous gaping.
Ngo TT, Ha T. Ngo TT, et al. Nucleic Acids Res. 2015 Apr 30;43(8):3964-71. doi: 10.1093/nar/gkv276. Epub 2015 Mar 30. Nucleic Acids Res. 2015. PMID: 25824950 Free PMC article. - Nucleosome composition regulates the histone H3 tail conformational ensemble and accessibility.
Morrison EA, Baweja L, Poirier MG, Wereszczynski J, Musselman CA. Morrison EA, et al. Nucleic Acids Res. 2021 May 7;49(8):4750-4767. doi: 10.1093/nar/gkab246. Nucleic Acids Res. 2021. PMID: 33856458 Free PMC article. - Nucleosome stability dramatically impacts the targeting of somatic hypermutation.
Kodgire P, Mukkawar P, North JA, Poirier MG, Storb U. Kodgire P, et al. Mol Cell Biol. 2012 May;32(10):2030-40. doi: 10.1128/MCB.06722-11. Epub 2012 Mar 5. Mol Cell Biol. 2012. PMID: 22393257 Free PMC article. - Characterization of chromatin accessibility patterns in different mouse cell types using machine learning methods at single-cell resolution.
Xu Y, Huang F, Guo W, Feng K, Zhu L, Zeng Z, Huang T, Cai YD. Xu Y, et al. Front Genet. 2023 Mar 1;14:1145647. doi: 10.3389/fgene.2023.1145647. eCollection 2023. Front Genet. 2023. PMID: 36936430 Free PMC article. - The histone chaperone Nap1 promotes nucleosome assembly by eliminating nonnucleosomal histone DNA interactions.
Andrews AJ, Chen X, Zevin A, Stargell LA, Luger K. Andrews AJ, et al. Mol Cell. 2010 Mar 26;37(6):834-42. doi: 10.1016/j.molcel.2010.01.037. Mol Cell. 2010. PMID: 20347425 Free PMC article.
References
- Richmond TJ, Davey CA. The structure of DNA in the nucleosome core. Nature. 2003;423:145–150. - PubMed
- Boeger H, Griesenbeck J, Strattan JS, Kornberg RD. Nucleosomes unfold completely at a transcriptionally active promoter. Mol Cell. 2003;11:1587–1598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM072306/GM/NIGMS NIH HHS/United States
- R01 GM58617/GM/NIGMS NIH HHS/United States
- R01 GM54692/GM/NIGMS NIH HHS/United States
- R01 GM058617/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 GM054692/GM/NIGMS NIH HHS/United States
- R01 GM054692-11/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources