Ciliated epithelial cell lifespan in the mouse trachea and lung - PubMed (original) (raw)
Ciliated epithelial cell lifespan in the mouse trachea and lung
Emma L Rawlins et al. Am J Physiol Lung Cell Mol Physiol. 2008 Jul.
Abstract
The steady-state turnover of epithelial cells in the lung and trachea is highly relevant to investigators who are studying endogenous stem cells, manipulating gene expression in vivo, or using viral vectors for gene therapy. However, the average lifetime of different airway epithelial cell types has not previously been assessed using currently available genetic techniques. Here, we use Cre/loxP genetic technology to indelibly label a random fraction of ciliated cells throughout the airways of a cohort of mice and follow them in vivo for up to 18 mo. We demonstrate that ciliated airway epithelial cells are a terminally differentiated population. Moreover, their average half-life of 6 mo in the trachea and 17 mo in the lung is much longer than previously available estimates, with significant numbers of labeled cells still present after 18 mo.
Figures
Fig. 1.
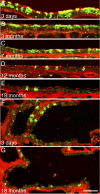
The percentage of lineage-labeled ciliated cells in the airways decreases over time. A_–_G: representative FOXJ1CreER 2T; Rosa26R-eYFP/+ frozen sections posttamoxifen. Green, anti-green fluorescent protein (GFP) (lineage label); red, anti-β-tubulin (cilia). A_–_E: trachea sections. F and G: terminal bronchiole sections. Bar = 50 μm in all panels.
Fig. 2.
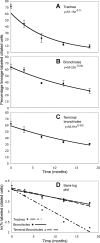
The percentage change of lineage-labeled ciliated cells fits to a first-order exponential decay function. A_–_C: the data points are the mean percentage of lineage-labeled ciliated cells ± SE. Exponential decay functions (curves) were fitted to these data by regression analysis. Half-life of the ciliated cells was estimated from the fitted equations to be 6 mo in the trachea (A), 17 mo in the bronchioles (B), and 14 mo in the terminal bronchioles (C). D: plotting the natural log of the percentage of lineage-labeled ciliated cells against time converts the exponential curves to straight lines.
Similar articles
- Basal cells as stem cells of the mouse trachea and human airway epithelium.
Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Rock JR, et al. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12771-5. doi: 10.1073/pnas.0906850106. Epub 2009 Jul 22. Proc Natl Acad Sci U S A. 2009. PMID: 19625615 Free PMC article. - A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells.
Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. Zhang Y, et al. Am J Respir Cell Mol Biol. 2007 May;36(5):515-9. doi: 10.1165/rcmb.2006-0475RC. Epub 2007 Jan 25. Am J Respir Cell Mol Biol. 2007. PMID: 17255554 Free PMC article. - Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli.
Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Perl AK, et al. Am J Respir Cell Mol Biol. 2005 Nov;33(5):455-62. doi: 10.1165/rcmb.2005-0180OC. Epub 2005 Jul 29. Am J Respir Cell Mol Biol. 2005. PMID: 16055670 Free PMC article. - Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair.
Rawlins EL, Okubo T, Que J, Xue Y, Clark C, Luo X, Hogan BL. Rawlins EL, et al. Cold Spring Harb Symp Quant Biol. 2008;73:291-5. doi: 10.1101/sqb.2008.73.037. Epub 2008 Nov 21. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19028985 Review. - Epithelial stem cells of the lung: privileged few or opportunities for many?
Rawlins EL, Hogan BL. Rawlins EL, et al. Development. 2006 Jul;133(13):2455-65. doi: 10.1242/dev.02407. Epub 2006 May 30. Development. 2006. PMID: 16735479 Review.
Cited by
- Ebola virus infection modeling and identifiability problems.
Nguyen VK, Binder SC, Boianelli A, Meyer-Hermann M, Hernandez-Vargas EA. Nguyen VK, et al. Front Microbiol. 2015 Apr 9;6:257. doi: 10.3389/fmicb.2015.00257. eCollection 2015. Front Microbiol. 2015. PMID: 25914675 Free PMC article. - Airway gene transfer in a non-human primate: lentiviral gene expression in marmoset lungs.
Farrow N, Miller D, Cmielewski P, Donnelley M, Bright R, Parsons DW. Farrow N, et al. Sci Rep. 2013;3:1287. doi: 10.1038/srep01287. Sci Rep. 2013. PMID: 23412644 Free PMC article. - Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air-liquid interface.
Pinkston R, Zaman H, Hossain E, Penn AL, Noël A. Pinkston R, et al. Respir Res. 2020 Oct 17;21(1):269. doi: 10.1186/s12931-020-01539-1. Respir Res. 2020. PMID: 33069224 Free PMC article. - Stem Cells in Lung Injury and Repair.
Chen F, Fine A. Chen F, et al. Am J Pathol. 2016 Oct;186(10):2544-50. doi: 10.1016/j.ajpath.2016.05.023. Epub 2016 Aug 11. Am J Pathol. 2016. PMID: 27524796 Free PMC article. Review. - In utero lung gene transfer using adeno-associated viral and lentiviral vectors in mice.
Joyeux L, Danzer E, Limberis MP, Zoltick PW, Radu A, Flake AW, Davey MG. Joyeux L, et al. Hum Gene Ther Methods. 2014 Jun;25(3):197-205. doi: 10.1089/hgtb.2013.143. Epub 2014 Apr 21. Hum Gene Ther Methods. 2014. PMID: 24660751 Free PMC article.
References
- Blenkinsopp WK Proliferation of respiratory tract epithelium in the rat. Exp Cell Res 46: 144–154, 1967. - PubMed
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24: 662–670, 2001. - PubMed
- Bowden DH Cell turnover in the lung. Am Rev Respir Dis 128: S46–S48, 1983. - PubMed
- Breuer R, Zajicek G, Christensen TG, Lucey EC, Snider GL. Cell kinetics of normal adult hamster bronchial epithelium in the steady state. Am J Respir Cell Mol Biol 2: 51–58, 1990. - PubMed
- Donnelly GM, Haack DG, Heird CS. Tracheal epithelium: cell kinetics and differentiation in normal rat tissue. Cell Tissue Kinet 15: 119–130, 1982. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases