The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis - PubMed (original) (raw)
The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis
John J Lehman et al. Am J Physiol Heart Circ Physiol. 2008 Jul.
Abstract
High-capacity mitochondrial ATP production is essential for normal function of the adult heart, and evidence is emerging that mitochondrial derangements occur in common myocardial diseases. Previous overexpression studies have shown that the inducible transcriptional coactivator peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha is capable of activating postnatal cardiac myocyte mitochondrial biogenesis. Recently, we generated mice deficient in PGC-1alpha (PGC-1alpha(-/-) mice), which survive with modestly blunted postnatal cardiac growth. To determine if PGC-1alpha is essential for normal cardiac energy metabolic capacity, mitochondrial function experiments were performed on saponin-permeabilized myocardial fibers from PGC-1alpha(-/-) mice. These experiments demonstrated reduced maximal (state 3) palmitoyl-l-carnitine respiration and increased maximal (state 3) pyruvate respiration in PGC-1alpha(-/-) mice compared with PGC-1alpha(+/+) controls. ATP synthesis rates obtained during maximal (state 3) respiration in permeabilized myocardial fibers were reduced for PGC-1alpha(-/-) mice, whereas ATP produced per oxygen consumed (ATP/O), a measure of metabolic efficiency, was decreased by 58% for PGC-1alpha(-/-) fibers. Ex vivo isolated working heart experiments demonstrated that PGC-1alpha(-/-) mice exhibited lower cardiac power, reduced palmitate oxidation, and increased reliance on glucose oxidation, with the latter likely a compensatory response. (13)C NMR revealed that hearts from PGC-1alpha(-/-) mice exhibited a limited capacity to recruit triglyceride as a source for lipid oxidation during beta-adrenergic challenge. Consistent with reduced mitochondrial fatty acid oxidative enzyme gene expression, the total triglyceride content was greater in hearts of PGC-1alpha(-/-) mice relative to PGC-1alpha(+/+) following a fast. Overall, these results demonstrate that PGC-1alpha is essential for the maintenance of maximal, efficient cardiac mitochondrial fatty acid oxidation, ATP synthesis, and myocardial lipid homeostasis.
Figures
Fig. 1.
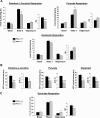
Respiration and ATP synthesis rates for permeabilized myocardial fibers and isolated mitochondria. A: respiration of saponin-permeabilized left ventricular (LV) fibers from hearts of 3.5-mo-old female peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α+/+ and PGC-1α−/− mice was measured as described in
materials and methods
. The respiration buffer contained 20 μM palmitoyl-
l
-carnitine (PC) and 5 mM malate for PC respiration, 10 mM pyruvate and 5 mM malate for pyruvate respiration, and 5 mM glutamate and 2 mM malate for glutamate respiration. Following measurements of basal respiration, state 3 (maximal ADP-stimulated) respiration was determined by exposing fibers to 1 mM ADP, with the subsequent determination of postoligomycin (uncoupled) respiration. The respiratory control (RC) quotient represents the following ratio: (state 3 respiration/postoligomycin respiration). Results are means ± SE; n = 10 for PC and pyruvate experiments and 6 for glutamate experiments. *P < 0.05 and **P < 0.01 compared with corresponding PGC-1α+/+ values. B: maximal ATP synthesis rates and efficiency of ATP synthesis [ATP produced per oxygen consumed (ATP/O)] in permeabilized LV fibers prepared in parallel from the same hearts used for respiration analysis. ATP/O represents the following ratio: (state 3 ATP synthesis rate/state 3 respiration rate). Results are means ± SE; n ≥ 8 for PC and pyruvate experiments and 5 for glutamate experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with corresponding PGC-1α+/+ values. C: respiration of mitochondria isolated from combined left and right cardiac ventricles of 4-mo-old sex-matched PGC-1α+/+ and PGC-1α−/− mice was measured in buffer containing 5 mM succinate and 10 μM rotenone as described in
materials and methods
. Following the assessment of basal respiration, state 3 (maximal ADP-stimulated) respiration was determined by exposing mitochondria to 350 μM ADP with the subsequent determination of postoligomycin (uncoupled) respiration. The RC quotient represents the following ratio: (state 3 respiration/postoligomycin respiration). Results are means ± SE; n = 6. *P < 0.05 compared with corresponding PGC-1α+/+ values.
Fig. 2.
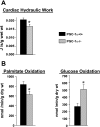
Alterations in substrate utilization and cardiac hydraulic work in isolated working hearts from PGC-1α−/− mice. A: an isolated mouse working heart perfusion was performed with hearts from 7- to 8-mo-old sex-matched male and female mice (as described in
materials and methods
). Results for cardiac hydraulic work are means ± SE; n ≥ 6. *P < 0.05 compared with corresponding PGC-1α+/+ values. B: to determine palmitate and glucose oxidation rates, trace amounts of [3H]palmitate (0.1 μCi/ml) and [U-14C]glucose (0.1 μCi/ml) were used in the isolated working heart perfusate. Results represent mean oxidation rates per gram ventricular dry weight ± SE; n ≥ 5. *P < 0.05 compared with corresponding PGC-1α+/+ values.
Fig. 3.
Altered mitochondrial cristal density in PGC-1α−/− cardiac ventricles. Representative electron micrographs of the cardiac LV apex obtained from normally fed 2-mo-old PGC-1α+/+ and PGC-1α−/− female mice are shown (representative of n ≥ 6 comparisons). Scale bars are shown to the right for the low (×30,000; top) and high (×120,000, bottom) magnifications.
Fig. 4.
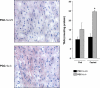
Assessment of cardiac ventricular neutral lipid and triglyceride (TAG) content. Representative histological sections stained with oil red O of the cardiac left ventricle from 4-mo-old PGC-1α+/+ and PGC-1α−/− mice following a 24-h fast are shown (left). The red droplets are indicative of neutral lipid accumulation. Mean cardiac ventricular TAG levels (right) were determined by two-dimensional electrospray ionization mass spectrometric (ESI/MS) analysis performed on myocardial lipid extracts from both fed and 24-h fasted PGC-1α+/+ and PGC-1α−/− mice (n ≥ 5). For the fast, 4-mo-old female mice were individually housed on wood chip bedding. Results are means ± SE. *P < 0.0001 compared with fasted PGC-1α+/+ values.
Fig. 5.
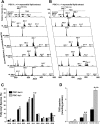
Two-dimensional ESI/MS fingerprint and quantitation of TAG molecular species of cardiac ventricular lipid extracts from fasted PGC-1α+/+ and PGC-1α−/− mice. A and B: two-dimensional ESI/MS analyses were performed on cardiac ventricular lipid extracts from 4-mo-old female PGC-1α+/+ (A) and PGC-1α−/− (B) mice following a 24-h fast as described in
materials and methods
. The top spectra depict the relative intensity of each TAG species compared with the T17:1 internal standard (IS) peak for TAGs. Below the relative intensity TAG spectrum, the subsequent horizontal rows depict the detailed analyses of TAG molecular species that contain the specific fatty acyl chain noted along the left axis. For example, the “NL 256.2 (16:0)” row indicates all the TAG molecular species containing at least one 16:0 fatty acyl chain. C: quantitation by ESI/MS of the relative abundance of fatty acyl species in TAG of cardiac ventricular lipid extracts from 24-h fasted PGC-1α+/+ and PGC-1α−/− mice. Results are means ± SE; n ≥ 5. *P < 0.05 and **P < 0.01 compared with fasted PGC-1α+/+ values. D: quantitation by ESI/MS of representative increased TAG species in cardiac ventricular lipid extracts from 24-h fasted PGC-1α+/+ and PGC-1α−/− mice. The increased TAG species in hearts of fasted PGC-1α−/− mice relative to PGC-1α+/+ mice included 16:0/16:0/16:0 TAG, 16:0/16:0/18:1 TAG, and 16:0/18:1/18:2 TAG. Results are means ± SE; n ≥ 5. *P < 0.05, **P < 0.01, and ***P < 0.0001 compared with corresponding PGC-1α+/+ values.
Similar articles
- Regulation of fatty acid metabolism by mTOR in adult murine hearts occurs independently of changes in PGC-1α.
Zhu Y, Soto J, Anderson B, Riehle C, Zhang YC, Wende AR, Jones D, McClain DA, Abel ED. Zhu Y, et al. Am J Physiol Heart Circ Physiol. 2013 Jul 1;305(1):H41-51. doi: 10.1152/ajpheart.00877.2012. Epub 2013 Apr 26. Am J Physiol Heart Circ Physiol. 2013. PMID: 23624629 Free PMC article. - PGC-1β deficiency accelerates the transition to heart failure in pressure overload hypertrophy.
Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. Riehle C, et al. Circ Res. 2011 Sep 16;109(7):783-93. doi: 10.1161/CIRCRESAHA.111.243964. Epub 2011 Jul 28. Circ Res. 2011. PMID: 21799152 Free PMC article. - The transcriptional coactivators, PGC-1α and β, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance.
Mitra R, Nogee DP, Zechner JF, Yea K, Gierasch CM, Kovacs A, Medeiros DM, Kelly DP, Duncan JG. Mitra R, et al. J Mol Cell Cardiol. 2012 Mar;52(3):701-10. doi: 10.1016/j.yjmcc.2011.10.010. Epub 2011 Oct 20. J Mol Cell Cardiol. 2012. PMID: 22080103 Free PMC article. - Fatty acid oxidation in the heart.
Grynberg A, Demaison L. Grynberg A, et al. J Cardiovasc Pharmacol. 1996;28 Suppl 1:S11-7. doi: 10.1097/00005344-199600003-00003. J Cardiovasc Pharmacol. 1996. PMID: 8891866 Review. - Nuclear receptor signaling and cardiac energetics.
Huss JM, Kelly DP. Huss JM, et al. Circ Res. 2004 Sep 17;95(6):568-78. doi: 10.1161/01.RES.0000141774.29937.e3. Circ Res. 2004. PMID: 15375023 Review.
Cited by
- The role of PGC-1 coactivators in aging skeletal muscle and heart.
Dillon LM, Rebelo AP, Moraes CT. Dillon LM, et al. IUBMB Life. 2012 Mar;64(3):231-41. doi: 10.1002/iub.608. Epub 2012 Jan 25. IUBMB Life. 2012. PMID: 22279035 Free PMC article. Review. - Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding.
Chambers KT, Chen Z, Crawford PA, Fu X, Burgess SC, Lai L, Leone TC, Kelly DP, Finck BN. Chambers KT, et al. PLoS One. 2012;7(12):e52645. doi: 10.1371/journal.pone.0052645. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285128 Free PMC article. - Tracing cardiac metabolism in vivo: one substrate at a time.
Taegtmeyer H. Taegtmeyer H. J Nucl Med. 2010 May 1;51 Suppl 1(0 1):80S-87S. doi: 10.2967/jnumed.109.068205. Epub 2010 Apr 15. J Nucl Med. 2010. PMID: 20395343 Free PMC article. Review. - Role of nuclear receptor SHP in metabolism and cancer.
Zhang Y, Hagedorn CH, Wang L. Zhang Y, et al. Biochim Biophys Acta. 2011 Aug;1812(8):893-908. doi: 10.1016/j.bbadis.2010.10.006. Epub 2010 Oct 20. Biochim Biophys Acta. 2011. PMID: 20970497 Free PMC article. Review. - Brain nuclear receptors and cardiovascular function.
Wang M, Yang Y, Xu Y. Wang M, et al. Cell Biosci. 2023 Jan 20;13(1):14. doi: 10.1186/s13578-023-00962-3. Cell Biosci. 2023. PMID: 36670468 Free PMC article. Review.
References
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005. - PubMed
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005. - PubMed
- Belke DD, Larsen TS, Lopaschuk GD, Severson DL. Glucose and fatty acid metabolism in the isolated working mouse heart. Am J Physiol Regul Integr Comp Physiol 277: R1210–R1217, 1999. - PubMed
- Benton CR, Han XX, Febbraio M, Graham TE, Bonen A. Inverse relationship between PGC-1α protein expression and triacylglycerol accumulation in rodent skeletal muscle. J Appl Physiol 100: 377–383, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL-3749244/HL/NHLBI NIH HHS/United States
- P30-DK-052574/DK/NIDDK NIH HHS/United States
- P30 DK056341-08/DK/NIDDK NIH HHS/United States
- R01-HL-73167/HL/NHLBI NIH HHS/United States
- P30-DK-056341/DK/NIDDK NIH HHS/United States
- R37 HL049244/HL/NHLBI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK056341-07/DK/NIDDK NIH HHS/United States
- R01-HL-058493/HL/NHLBI NIH HHS/United States
- K08-AG-024844/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases