The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors - PubMed (original) (raw)
Comparative Study
. 2008 Jun 1;121(11):1907-15.
doi: 10.1242/jcs.029397.
Affiliations
- PMID: 18492793
- PMCID: PMC2637114
- DOI: 10.1242/jcs.029397
Comparative Study
The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors
Bryan L Krock et al. J Cell Sci. 2008.
Abstract
Defects in protein transport within vertebrate photoreceptors can result in photoreceptor degeneration. In developing and mature photoreceptors, proteins targeted to the outer segment are transported through the connecting cilium via the process of intraflagellar transport (IFT). In studies of vertebrate IFT, mutations in any component of the IFT particle typically abolish ciliogenesis, suggesting that IFT proteins are equally required for IFT. To determine whether photoreceptor outer segment formation depends equally on individual IFT proteins, we compared the retinal phenotypes of IFT57 and IFT88 mutant zebrafish. IFT88 mutants failed to form outer segments, whereas IFT57 mutants formed short outer segments with reduced amounts of opsin. Our phenotypic analysis revealed that IFT57 is not essential for IFT, but is required for efficient IFT. In co-immunoprecipitation experiments from whole-animal extracts, we determined that kinesin II remained associated with the IFT particle in the absence of IFT57, but IFT20 did not. Additionally, kinesin II did not exhibit ATP-dependent dissociation from the IFT particle in IFT57 mutants. We conclude that IFT20 requires IFT57 to associate with the IFT particle and that IFT57 and/or IFT20 mediate kinesin II dissociation.
Figures
Fig. 1
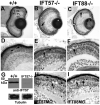
Histological sections of 4 dpf wild-type, IFT57 and IFT88 mutant and morphant retinas. (A) Wild-type retinas at 4 dpf are fully laminated, and the outer nuclear layer (ONL), inner nuclear layer (INL) and retinal ganglion cells (RGC) are present. The outer plexiform layer (OPL) and the inner plexiform layer (IPL) are easily observable. (B,C) Lamination and cellular differentiation are unaffected in both IFT57 and IFT88 mutants; however, acellular holes (arrows) in the ONL are observed. (D) High-magnification images of wild-type retinas showing photoreceptor outer segments (arrowheads). Outer segments are not observed in IFT88 mutants (C); however, short outer segments are observed in IFT57 mutants (arrowhead). (G) Western blot analysis of IFT57 mutants at 4 dpf. No IFT57 protein is observed in IFT57 mutants (upper blot, lane 2). Blots probed with anti-acetylated tubulin serve as a loading control (lower blot). (H,I) Histological analysis of IFT57 and IFT88 morphants phenocopy the IFT57 and IFT88 mutations, respectively. Arrowheads in D,E,H indicate outer segments and arrows in E,H,I indicate acellular holes or pyknotic nuclei. Scale bar: 100 μm in A-C; 20 μm in D-F,H,I.
Fig. 2
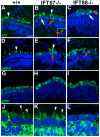
Immunohistochemical analysis of wild type and IFT mutants. Retinal cryosections of 4 dpf larvae were stained with 1D1, an antibody that labels rhodopsin, with BOPS, an antibody against blue cone opsin, and with ZPR1, an antibody that recognizes an unknown epitope of red-green double cones. In addition, anti-acetylated tubulin (AT) was used to label microtubules and visualize cilia. In all panels, immunolabel is shown in green and nuclei are counterstained with DAPI (blue). (A) Wild-type photoreceptors have rhodopsin localized almost exclusively to the outer segment (arrowhead). (B) IFT57 mutants display significant rhodopsin localization to the outer segment (arrowhead), but punctate areas of rhodopsin mislocalization (inset, red arrowhead) are observed, as well as mislocalization of rhodopsin through the plasma membrane (red arrow). (C) IFT88 mutant photoreceptors have rhodopsin completely mislocalized throughout the plasma membrane (red arrow) and both IFT mutants exhibit cell death in the photoreceptor layer, as indicated by condensed and bright DAPI-labeled nuclei of pyknotic cells (white arrow). (D-F) Blue opsin also localizes to the outer segments of blue cones. IFT57 and IFT88 mutants exhibit a similar pattern of mislocalization with blue cone opsin as seen with rhodopsin. (G-I) ZPR1 labeling indicates red-green double-cone morphology at 4 dpf in wild type. IFT57 and IFT88 mutants have shorter cones when measured in the apical-basal axis, and adopt an abnormal morphology. (J-L) Anti-acetylated tubulin stains microtubules in the connecting cilia (arrowheads) that project apically from the cell body in both wild type and IFT57 mutants, but not in IFT88 mutants. Scale bar: 10 μm.
Fig. 3
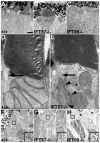
Transmission electron microscopy of 4 dpf wild-type, IFT57 and IFT88 mutant retinas. (A-C) Low-magnification electron micrographs of wild-type and mutant retinas, illustrating the outer segment (OS), inner segment (IS) and nucleus (N). Wild type and IFT57 mutants both exhibit photoreceptor outer segments, but IFT88 mutants lack these structures. (D,E) High-magnification micrographs of wild-type and IFT57 mutant retinas. Wild-type photoreceptors exhibit well-organized disk stacking within the outer segment, and the connecting cilium (black arrow) is observable. IFT57 mutants also demonstrate normal disk stacking within the outer segment, and the base of the connecting cilium (black arrow) is evident. An accumulation of vesicles (black arrowheads) is observed at the base of the cilium. Asterisk indicates centrioles oriented at right angles within the photoreceptor. (F-H) Immunogold labeling of rhodopsin in wild-type retinas shows dense labeling within the outer segment. Outer segments are outlined by broken lines and insets are magnified views of the boxed area in each panel, which illustrate gold particle density. IFT57 mutant outer segments demonstrate a lower density of label when compared with wild type, whereas rhodopsin localizes most densely to the apical part of the photoreceptor in IFT88 mutants. Scale bar: 2 μm in A-C; 200 nm in D,E; 0.75 μm in F; 1 μm in G,H.
Fig. 4
Quantification of photoreceptor outer segment length and rhodopsin staining density within the outer segment in wild type and IFT57 mutants. (A) IFT57 mutant photoreceptor outer segments are reduced in length by 75% when compared with age-matched wild-type photoreceptors. Data were taken from retinas of four animals for both wild type and IFT mutants. (B) Photoreceptor outer segments in IFT57 mutants contain 59% less rhodopsin. Staining density was determined by counting colloidal gold particles in a random 0.25 μm2 region of rod outer segments with each data point obtained from a unique outer segment. Data were obtained from retinas of four animals for both wild type and IFT57 mutants. (A) P<0.01, (B) P<0.0001, as determined by a Student's _t_-test.
Fig. 5
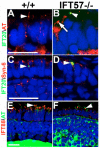
IFT20 and IFT88 localize to the base of the cilium in IFT57 mutant rods. (A) Wild-type rods expressing an IFT20-GFP transgene exhibit IFT20 localization at the base of the cilium (acetylated tubulin), where it colocalizes with acetylated tubulin (yellow; arrowhead). As the embryos do not carry an integrated transgene, only a small number of rod photoreceptors in the retina exhibit expression. (B) Loss of IFT57 does not affect IFT20-GFP localization to connecting cilia (arrowhead). Arrow indicates the connecting cilia in a neighboring cell that does not express the IFT20-GFP transgene. (C,D) In the presence or absence of IFT57, IFT20-GFP localized to the Golgi apparatus, where it colocalizes with syntaxin 6 (arrowhead). (E) IFT88 localizes to the base of the connecting cilium (arrowhead) in wild-type photoreceptors as well as in (F) IFT57 mutant photoreceptors. Abbreviations: AT, acetylated tubulin; Syn-6, syntaxin 6. Scale bar: 10 μm in A,B,E,F; 40 μm in C,D.
Fig. 6
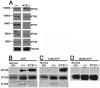
Biochemical analysis of IFT57 mutants. (A) Western blot of lysates generated from the larval heads of 4 dpf wild type and IFT57 mutants. Mutation of IFT57 does not significantly alter the abundance of any other IFT protein other than IFT57. (B) Lysates of wild type (+/+) or IFT57 mutants (IFT57−/−) were incubated with rabbit antibodies against zebrafish IFT88 or normal rabbit IGG as a negative control. Immunoprecipitates were subsequently blotted with antibodies against KIF3A, IFT52 and IFT20. In IFT57 mutants, IFT20 is not precipitated along with the IFT complex, whereas more KIF3A is precipitated. (C) The addition of 1 mM ATP to lysates blocks the association of KIF3A with the IFT particle in wild-type lysates but not in IFT57 lysates. Lysates were prepared as described above, and then ATP was added to a final concentration of 1 mM prior to the addition of IFT88 antibody and precipitation. (D) The addition of 10 mM ATP blocks the association of KIF3A with the IFT particle in both wild-type and mutant lysates. Asterisks indicate IGG heavy chain band from precipitating antibody on immunoblots.
Fig. 7
IFT57 and IFT20 mediate the ATP-dependent dissociation of kinesin. (A) A model describing the nature of protein interactions within the IFT complex. IFT57 tethers IFT20 to the IFT particle, while kinesin binds to the IFT particle through an unknown entity. IFT20 physically interacts with KIF3B and mediates the ATP-dependent dissociation of kinesin. (B) In the absence of IFT57, IFT20 can no longer associate with the IFT particle. However, the interaction of kinesin with the IFT particle is stabilized by loss of IFT57 and IFT20 from the IFT particle.
Similar articles
- Loss of ift122, a Retrograde Intraflagellar Transport (IFT) Complex Component, Leads to Slow, Progressive Photoreceptor Degeneration Due to Inefficient Opsin Transport.
Boubakri M, Chaya T, Hirata H, Kajimura N, Kuwahara R, Ueno A, Malicki J, Furukawa T, Omori Y. Boubakri M, et al. J Biol Chem. 2016 Nov 18;291(47):24465-24474. doi: 10.1074/jbc.M116.738658. Epub 2016 Sep 28. J Biol Chem. 2016. PMID: 27681595 Free PMC article. - IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia.
Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. Baker SA, et al. J Biol Chem. 2003 Sep 5;278(36):34211-8. doi: 10.1074/jbc.M300156200. Epub 2003 Jun 23. J Biol Chem. 2003. PMID: 12821668 - Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants.
Sukumaran S, Perkins BD. Sukumaran S, et al. Vision Res. 2009 Feb;49(4):479-89. doi: 10.1016/j.visres.2008.12.009. Epub 2009 Jan 21. Vision Res. 2009. PMID: 19136023 Free PMC article. - The Role of Intraflagellar Transport in the Photoreceptor Sensory Cilium.
Taub DG, Liu Q. Taub DG, et al. Adv Exp Med Biol. 2016;854:627-33. doi: 10.1007/978-3-319-17121-0_83. Adv Exp Med Biol. 2016. PMID: 26427468 Review. - Review: Intraflagellar transport proteins in the retina.
Kannabiran C. Kannabiran C. Mol Vis. 2020 Oct 4;26:652-660. eCollection 2020. Mol Vis. 2020. PMID: 33088169 Free PMC article. Review.
Cited by
- Cilia/Ift protein and motor -related bone diseases and mouse models.
Yuan X, Yang S. Yuan X, et al. Front Biosci (Landmark Ed). 2015 Jan 1;20(3):515-55. doi: 10.2741/4323. Front Biosci (Landmark Ed). 2015. PMID: 25553465 Free PMC article. Review. - The Formation and Renewal of Photoreceptor Outer Segments.
Xu J, Zhao C, Kang Y. Xu J, et al. Cells. 2024 Aug 15;13(16):1357. doi: 10.3390/cells13161357. Cells. 2024. PMID: 39195247 Free PMC article. Review. - Protein sorting, targeting and trafficking in photoreceptor cells.
Pearring JN, Salinas RY, Baker SA, Arshavsky VY. Pearring JN, et al. Prog Retin Eye Res. 2013 Sep;36:24-51. doi: 10.1016/j.preteyeres.2013.03.002. Epub 2013 Apr 3. Prog Retin Eye Res. 2013. PMID: 23562855 Free PMC article. Review. - Molecular complexes that direct rhodopsin transport to primary cilia.
Wang J, Deretic D. Wang J, et al. Prog Retin Eye Res. 2014 Jan;38:1-19. doi: 10.1016/j.preteyeres.2013.08.004. Epub 2013 Oct 14. Prog Retin Eye Res. 2014. PMID: 24135424 Free PMC article. Review. - The route of the visual receptor rhodopsin along the cilium.
Chadha A, Volland S, Baliaouri NV, Tran EM, Williams DS. Chadha A, et al. J Cell Sci. 2019 May 15;132(10):jcs229526. doi: 10.1242/jcs.229526. J Cell Sci. 2019. PMID: 30975916 Free PMC article.
References
- Amsterdam A, Hopkins N. Retrovirus-mediated insertional mutagenesis in zebrafish. Methods Cell Biol. 1999;60:87–98. - PubMed
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J. Biol. Chem. 2003;278:34211–34218. - PubMed
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat. Genet. 2007;39:727–729. - PubMed
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Dryja TP. Ocular findings in patients with autosomal dominant retinitis pigmentosa and rhodopsin, proline-347-leucine. Am. J. Ophthalmol. 1991;111:614–623. - PubMed
- Besharse JC, Horst CJ. The Photoreceptor Connecting Cilium. A Model for the Transition Zone. Plenum; New York: 1990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases