Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice - PubMed (original) (raw)
Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice
Zerong You et al. J Cereb Blood Flow Metab. 2008 Sep.
Abstract
Necroptosis is a newly identified type of programmed necrosis initiated by the activation of tumor necrosis factor alpha (TNFalpha)/Fas. Necrostatin-1 is a specific inhibitor of necroptosis that reduces ischemic tissue damage in experimental stroke models. We previously reported decreased tissue damage and improved functional outcome after controlled cortical impact (CCI) in mice deficient in TNFalpha and Fas. Hence, we hypothesized that necrostatin-1 would reduce histopathology and improve functional outcome after CCI in mice. Compared with vehicle-/inactive analog-treated controls, mice administered necrostatin-1 before CCI had decreased propidium iodide-positive cells in the injured cortex and dentate gyrus (6 h), decreased brain tissue damage (days 14, 35), improved motor (days 1 to 7), and Morris water maze performance (days 8 to 14) after CCI. Improved spatial memory was observed even when drug was administered 15 mins after CCI. Necrostatin-1 treatment did not reduce caspase-3-positive cells in the dentate gyrus or cortex, consistent with a known caspase-independent mechanism of necrostatin-1. However, necrostatin-1 reduced brain neutrophil influx and microglial activation at 48 h, suggesting a novel anti-inflammatory effect in traumatic brain injury (TBI). The data suggest that necroptosis plays a significant role in the pathogenesis of cell death and functional outcome after TBI and that necrostatin-1 may have therapeutic potential for patients with TBI.
Figures
Figure 1
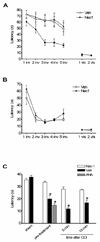
Brain lesion volume is reduced in Necrostatin-1 treated mice after controlled cortical impact (CCI). (A) Pretreatment with necrostatin-1 reduced lesion volume at 14 days after CCI compared to treatment with vehicle (Veh) or inactive analogue (ANA)(p < 0.001 ANOVA, *p < 0.05 versus ANA or Veh). Pretreatment with necrostatin-1 also reduced lesion size assessed at 35 days (*p < 0.05). For 14 days, n = 7–16/group; for 35 days, n = 4/group). (B) Necrostatin-1 was administered after CCI at the times indicated. Post injury treatment with necrostatin-1 reduced lesion size compared to vehicle when administered at 5 or 15 min but not 30 min after CCI (*p < 0.05, n = 4–5/group).
Figure 2
Reduced motor deficits after controlled cortical impact (CCI) in Necrostatin-1 treated mice. Vestibulo-motor function assessed by the wire grip test was similar among all groups prior to CCI. Post injury motor function was significantly improved in mice administered necrostatin-1 (Nec-1) compared to inactive analogue (ANA) or vehicle (Veh) (p < 0.05 for group effect, n=8/group). (B) Administration of necrostatin-1 (n = 5) to mice 5 min after CCI did not affect motor performance vs. vehicle treated animals (n = 9).
Figure 3
Necrostatin-1 treatment improves Morris water maze (MWM) performance after controlled cortical impact (CCI). (A) Morris water maze performance in naive adult animals administered necrostatin-1 (Nec-1) or vehicle (Veh). No difference in performance was observed between uninjured Veh- and Nec-1-treated mice in hidden or visible platform trials, or in probe trials (n=4/group). (B) Performance in the hidden platform trials was significantly improved in animals pretreated with Necrostatin-1 versus inactive analogue (ANA) or vehicle (Veh) (p < 0.05 for group effect, n = 7/group). (C) Probe trial performance in mice administered Necrostatin-1 (Nec-1) vs. Veh or ANA. No effect of drug treatment was observed in sham injured mice on probe trial performance, however injured mice administered Nec-1 before or at 5 or 15 minutes after CCI had improved performance vs. vehicle-treated animals (*p < 0.05), n= 4–9/group).
Figure 4
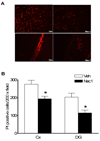
Pre-treatment with necrostatin-1 (Nec-1) reduces propidium iodide (PI)-positive cells at 6 h after controlled cortical impact (CCI) in injured cortex and hippocampus. (A) Representative photomicrographs showing reduced numbers of PI-positive cells in cortical and dentate gyrus brain regions after CCI in Nec-1 and vehicle-treated mice. Magnification × 200. (B) Quantitation of PI-positive cells in injured cortex and dentate gyrus. *p < 0.01 vs. vehicle treated animals (n = 12/group).
Figure 5
No effect of necrostatin-1 on caspase-3-positive cells after controlled cortical impact. Necrostatin-1 or vehicle was administered to mice (n = 7–9/group) immediately before CCI and caspase-3 positive cells were quantitated in injured cortex and dentate gyrus at 48 h. (A) Representative photomicrographs showing similar numbers of caspase-3-positive cells in dentate gyrus after CCI in Necrostatin-1 (Nec-1) and vehicle-treated (Veh) mice. (B) Quantitation of caspase-3-positive cells in injured cortex and dentate gyrus.
Figure 6
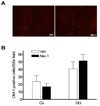
Pre-treatment with necrostatin-1 reduces neuroinflammation assessed at 48 h after controlled cortical impact. (A) Representative photomicrographs (top panels) showing neutrophil accumulation in injured cortex in mice administered vehicle (Veh) or necrostatin-1 (Nec-1). Bottom panels show quantitation of neutrophils in injured cortices of Veh and Nec-1 treated mice. * p < 0.05 vs. Veh (n = 10–11/group). (B) Marked reduction in microglial activation in mice administered necrostatin-1 vs. vehicle prior to controlled cortical impact. Upper panels show representative immunohistochemical staining using the microglial specific marker IBA-1. The graph in the lower panel shows quantitation of microglial activation using image analysis software. *p < 0.05 vs. vehicle (n = 7–9/group). Magnification × 200 in all photomicrographs.
Similar articles
- Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model.
Wang YQ, Wang L, Zhang MY, Wang T, Bao HJ, Liu WL, Dai DK, Zhang L, Chang P, Dong WW, Chen XP, Tao LY. Wang YQ, et al. Neurochem Res. 2012 Sep;37(9):1849-58. doi: 10.1007/s11064-012-0791-4. Epub 2012 Jun 27. Neurochem Res. 2012. PMID: 22736198 - TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice.
Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. Bermpohl D, et al. J Cereb Blood Flow Metab. 2007 Nov;27(11):1806-18. doi: 10.1038/sj.jcbfm.9600487. Epub 2007 Apr 4. J Cereb Blood Flow Metab. 2007. PMID: 17406655 - Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway.
Su X, Wang H, Kang D, Zhu J, Sun Q, Li T, Ding K. Su X, et al. Neurochem Res. 2015 Apr;40(4):643-50. doi: 10.1007/s11064-014-1510-0. Epub 2015 Jan 10. Neurochem Res. 2015. PMID: 25576092 - The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury.
Liao S, Apaijai N, Chattipakorn N, Chattipakorn SC. Liao S, et al. Arch Biochem Biophys. 2020 Nov 30;695:108629. doi: 10.1016/j.abb.2020.108629. Epub 2020 Oct 14. Arch Biochem Biophys. 2020. PMID: 33068524 Review.
Cited by
- Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model.
Wang YQ, Wang L, Zhang MY, Wang T, Bao HJ, Liu WL, Dai DK, Zhang L, Chang P, Dong WW, Chen XP, Tao LY. Wang YQ, et al. Neurochem Res. 2012 Sep;37(9):1849-58. doi: 10.1007/s11064-012-0791-4. Epub 2012 Jun 27. Neurochem Res. 2012. PMID: 22736198 - RIP1 Inhibition Rescues from LPS-Induced RIP3-Mediated Programmed Cell Death, Distributed Energy Metabolism and Spatial Memory Impairment.
Nikseresht S, Khodagholi F, Nategh M, Dargahi L. Nikseresht S, et al. J Mol Neurosci. 2015 Oct;57(2):219-30. doi: 10.1007/s12031-015-0609-3. Epub 2015 Jul 9. J Mol Neurosci. 2015. PMID: 26156201 - The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities.
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. Lewerenz J, et al. Antioxid Redox Signal. 2013 Feb 10;18(5):522-55. doi: 10.1089/ars.2011.4391. Epub 2012 Aug 3. Antioxid Redox Signal. 2013. PMID: 22667998 Free PMC article. Review. - Arc silence aggravates traumatic neuronal injury via mGluR1-mediated ER stress and necroptosis.
Chen T, Zhu J, Wang YH, Hang CH. Chen T, et al. Cell Death Dis. 2020 Jan 2;11(1):4. doi: 10.1038/s41419-019-2198-5. Cell Death Dis. 2020. PMID: 31919348 Free PMC article. - Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis.
Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J, Lipinski MM. Liu S, et al. Cell Death Dis. 2018 May 1;9(5):476. doi: 10.1038/s41419-018-0469-1. Cell Death Dis. 2018. PMID: 29686269 Free PMC article.
References
- Aoyama N, Katayama Y, Kawamata T, Maeda T, Mori T, Yamamoto T, Kikuchi T, Uwahodo Y. Effects of antioxidant, OPC-14117, on secondary cellular damage and behavioral deficits following cortical contusion in the rat. Brain Res. 2002;934:117–124. - PubMed
- Beer R, Franz G, Schopf M, Reindl M, Zelger B, Schmutzhard E, Poewe W, Kampfl A. Expression of Fas and Fas ligand after experimental traumatic brain injury in the rat. J Cereb Blood Flow Metab. 2000;20:669–677. - PubMed
- Bermpohl D, You Z, Korsmeyer SJ, Moskowitz MA, Whalen MJ. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab. 2006;26:625–633. - PubMed
- Bermpohl DYZ, Lo EH, Kim H, Moskowitz MA, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007 In press. - PubMed
- Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem. 2000;74:740–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS067497/NS/NINDS NIH HHS/United States
- R01NS47447/NS/NINDS NIH HHS/United States
- P30 NS045776/NS/NINDS NIH HHS/United States
- R01 NS047447/NS/NINDS NIH HHS/United States
- P30NS45776/NS/NINDS NIH HHS/United States
- R01 NS061255/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous