Aquaporin-4-deficient mice have increased extracellular space without tortuosity change - PubMed (original) (raw)
Aquaporin-4-deficient mice have increased extracellular space without tortuosity change
Xiaoming Yao et al. J Neurosci. 2008.
Abstract
Aquaporin-4 (AQP4) is the major water channel expressed at fluid-tissue barriers throughout the brain and plays a crucial role in cerebral water balance. To assess whether these channels influence brain extracellular space (ECS) under resting physiological conditions, we used the established real-time iontophoresis method with tetramethylammonium (TMA(+)) to measure three diffusion parameters: ECS volume fraction (alpha), tortuosity (lambda), and TMA(+) loss (k'). In vivo measurements were performed in the somatosensory cortex of AQP4-deficient (AQP4(-/-)) mice and wild-type controls with matched age. Mice lacking AQP4 showed a 28% increase in alpha (0.23 +/- 0.007 vs 0.18 +/- 0.003) with no differences in lambda (1.62 +/- 0.04 vs 1.61 +/- 0.02) and k' (0.0045 +/- 0.0001 vs 0.0031 +/- 0.0009 s(-1)). Additional recordings in brain slices showed similarly elevated alpha in AQP4(-/-) mice, and no differences in lambda and k' between the two genotypes. This is the first direct comparison of ECS properties in adult mice lacking AQP4 water channels with wild-type animals and demonstrates a significant enlargement of the volume fraction but no difference in hindrance to TMA(+) diffusion, expressed as tortuosity. These findings provide direct evidence for involvement of AQP4 in modulation of the ECS volume fraction and provide a basis for future modeling of water and ion transport in the CNS.
Figures
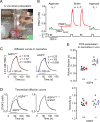
Figure 1.
Diffusion of TMA+ in the somatosensory neocortex of AQP4+/+ and AQP4−/− mice in vivo. A, Photograph of recording arrangement for RTI-TMA diffusion measurements. The mouse head was immobilized in a stereotaxic frame. A micromanipulator positioned the microelectrodes that were glued with dental cement into an array with fixed intertip distance. The grounding electrode and the temperature probe were positioned nearby. See Materials and Methods for details. B, Sequence of diffusion curves recorded in dilute agarose gel and neocortex of AQP4+/+ mice. Several records were obtained in agarose before and after brain measurements. See Results for details. C, Examples of TMA+ diffusion curves in AQP4+/+ and AQP4−/− mice. The measurements were done at 37°C, where _D_37 = 1.31 × 10−5 cm2/s. TMA+ pulse was applied for 50 s (horizontal bar), r was 144 and 124 μm for AQP4+/+ and AQP4−/−, respectively, and _n_t was 0.33 for both records. Recorded curves (red and blue) are superimposed with corresponding theoretical curves obtained from fitting procedure (dashed and dotted). D, Diffusion curves obtained in AQP4+/+ compared with those in AQP4−/− mice by generating theoretical curves based on the average α, λ, and k′ values (Table 1 and E) with the following parameters: temperature, 37°C; bias current, + 20 nA; main current, +120 nA for 50 s; r = 130 μm; _n_t = 0.4. The AQP4−/− theoretical curve had smaller amplitude than that for AQP4+/+ animals (left), reflecting a larger α, but both curves had similar shapes (right), indicating similar values of λ. E, Scatter plots of whole datasets. Each small circle represents the average from one animal. Large circles are mean ± SEM values; *p < 0.001.
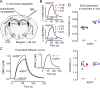
Figure 2.
Diffusion of TMA+ in the somatosensory neocortex (S1) of AQP4+/+ and AQP4−/− mice in vitro. A, A schematic of coronal brain slice with two independent microelectrodes positioned in S1 [Franklin and Paxinos (2008), their Figure 43, modified with permission]. B, Representative TMA+ diffusion curves in AQP4+/+ (top) and AQP4−/− (bottom) mice. The measurements were done at 34 and 32°C in AQP4+/+ and AQP4−/− mice, respectively; _D_34 = 1.24 × 10−5 cm2/s and _D_32 = 1.19 × 10−5 cm2/s. TMA+ pulse was applied for 50 s (horizontal bar), r was 130 μm, and _n_t was 0.39 and 0.48 for AQP4+/+ and AQP4−/− mice, respectively. Actual AQP4+/+ record was taken with 60 nA current but scaled to 120 nA for consistency. In both panels, recorded curves (solid red or blue lines) are superimposed with corresponding theoretical curves obtained from fitting procedure (dashed and dotted). C, As in Figure 1, to compare the diffusion properties in AQP4+/+ and AQP4−/− mice, two theoretical curves were generated from average α, λ, and k′ values (Table 1 and D) with the same parameters as in Figure 1, except temperature was 34°C. As in Figure 1, TMA+ diffusion curve in AQP4−/− mice has smaller amplitude than AQP4+/+ but similar shape (inset). D, Scatter plots of entire datasets showing a significant increase in the volume fraction in AQP4−/− (top) but no change in the tortuosity (bottom). Each small circle represents the average from a single slice. Large circles are mean ± SEM values; *p < 0.001.
Similar articles
- Enlarged extracellular space of aquaporin-4-deficient mice does not enhance diffusion of Alexa Fluor 488 or dextran polymers.
Xiao F, Hrabetová S. Xiao F, et al. Neuroscience. 2009 Jun 16;161(1):39-45. doi: 10.1016/j.neuroscience.2009.03.017. Epub 2009 Mar 19. Neuroscience. 2009. PMID: 19303428 Free PMC article. - The impact of alpha-syntrophin deletion on the changes in tissue structure and extracellular diffusion associated with cell swelling under physiological and pathological conditions.
Dmytrenko L, Cicanic M, Anderova M, Vorisek I, Ottersen OP, Sykova E, Vargova L. Dmytrenko L, et al. PLoS One. 2013 Jul 5;8(7):e68044. doi: 10.1371/journal.pone.0068044. Print 2013. PLoS One. 2013. PMID: 23861848 Free PMC article. - The role of aquaporin-4 and transient receptor potential vaniloid isoform 4 channels in the development of cytotoxic edema and associated extracellular diffusion parameter changes.
Chmelova M, Sucha P, Bochin M, Vorisek I, Pivonkova H, Hermanova Z, Anderova M, Vargova L. Chmelova M, et al. Eur J Neurosci. 2019 Jul;50(1):1685-1699. doi: 10.1111/ejn.14338. Epub 2019 Feb 8. Eur J Neurosci. 2019. PMID: 30633415 - Diffusion in brain extracellular space.
Syková E, Nicholson C. Syková E, et al. Physiol Rev. 2008 Oct;88(4):1277-340. doi: 10.1152/physrev.00027.2007. Physiol Rev. 2008. PMID: 18923183 Free PMC article. Review. - New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice.
Manley GT, Binder DK, Papadopoulos MC, Verkman AS. Manley GT, et al. Neuroscience. 2004;129(4):983-91. doi: 10.1016/j.neuroscience.2004.06.088. Neuroscience. 2004. PMID: 15561413 Review.
Cited by
- Regulation and Function of AQP4 in the Central Nervous System.
Assentoft M, Larsen BR, MacAulay N. Assentoft M, et al. Neurochem Res. 2015 Dec;40(12):2615-27. doi: 10.1007/s11064-015-1519-z. Epub 2015 Jan 29. Neurochem Res. 2015. PMID: 25630715 Review. - CrossTalk opposing view: Going against the flow: interstitial solute transport in brain is diffusive and aquaporin-4 independent.
Smith AJ, Verkman AS. Smith AJ, et al. J Physiol. 2019 Sep;597(17):4421-4424. doi: 10.1113/JP277636. Epub 2019 Aug 6. J Physiol. 2019. PMID: 31389038 Free PMC article. No abstract available. - Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice.
Igarashi H, Tsujita M, Kwee IL, Nakada T. Igarashi H, et al. Neuroreport. 2014 Jan 8;25(1):39-43. doi: 10.1097/WNR.0000000000000042. Neuroreport. 2014. PMID: 24231830 Free PMC article. - Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet.
Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare Ø, Laake P, Klungland A, Thorén AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Haj-Yasein NN, et al. Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17815-20. doi: 10.1073/pnas.1110655108. Epub 2011 Oct 11. Proc Natl Acad Sci U S A. 2011. PMID: 21990350 Free PMC article. - Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice.
Hirt L, Fukuda AM, Ambadipudi K, Rashid F, Binder D, Verkman A, Ashwal S, Obenaus A, Badaut J. Hirt L, et al. J Cereb Blood Flow Metab. 2017 Jan;37(1):277-290. doi: 10.1177/0271678X15623290. Epub 2016 Jan 14. J Cereb Blood Flow Metab. 2017. PMID: 26767580 Free PMC article.
References
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci USA. 2003;100:13615–13620. - PMC - PubMed
- Anděrová M, Kubinová S, Mazel T, Chvátal A, Eliasson C, Pekny M, Syková E. Effect of elevated K+, hypotonic stress, and cortical spreading depression on astrocyte swelling in GFAP-deficient mice. Glia. 2001;35:189–203. - PubMed
- Binder DK, Oshio K, Ma T, Verkman AS, Manley GT. Increased seizure threshold in mice lacking aquaporin-4 water channels. NeuroReport. 2004a;15:259–262. - PubMed
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS028642/NS/NINDS NIH HHS/United States
- R01 NS047557-04/NS/NINDS NIH HHS/United States
- R01 NS047557-01A1/NS/NINDS NIH HHS/United States
- R01 NS050173-04/NS/NINDS NIH HHS/United States
- R56 NS047557/NS/NINDS NIH HHS/United States
- R01 NS028642-17/NS/NINDS NIH HHS/United States
- R01 NS050173-03/NS/NINDS NIH HHS/United States
- NS28642/NS/NINDS NIH HHS/United States
- R01 NS028642-11A1/NS/NINDS NIH HHS/United States
- U10 NS058931/NS/NINDS NIH HHS/United States
- R01 NS028642-14/NS/NINDS NIH HHS/United States
- R01 NS028642-10/NS/NINDS NIH HHS/United States
- R01 NS050173-02/NS/NINDS NIH HHS/United States
- R01 NS028642-18/NS/NINDS NIH HHS/United States
- R01 NS047557-02/NS/NINDS NIH HHS/United States
- R01 NS028642-13/NS/NINDS NIH HHS/United States
- R01 NS028642-15/NS/NINDS NIH HHS/United States
- NS058931/NS/NINDS NIH HHS/United States
- NS050173/NS/NINDS NIH HHS/United States
- R01 NS050173/NS/NINDS NIH HHS/United States
- R01 NS050173-01A1/NS/NINDS NIH HHS/United States
- R01 NS028642-12/NS/NINDS NIH HHS/United States
- R01 NS047557-06/NS/NINDS NIH HHS/United States
- R01 NS028642-16/NS/NINDS NIH HHS/United States
- NS047557/NS/NINDS NIH HHS/United States
- R01 NS047557/NS/NINDS NIH HHS/United States
- R01 NS047557-05/NS/NINDS NIH HHS/United States
- R01 NS047557-03/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases