DLC1 suppresses distant dissemination of human hepatocellular carcinoma cells in nude mice through reduction of RhoA GTPase activity, actin cytoskeletal disruption and down-regulation of genes involved in metastasis - PubMed (original) (raw)
DLC1 suppresses distant dissemination of human hepatocellular carcinoma cells in nude mice through reduction of RhoA GTPase activity, actin cytoskeletal disruption and down-regulation of genes involved in metastasis
Xiaoling Zhou et al. Int J Oncol. 2008 Jun.
Abstract
The process of cell dissemination from the primary tumors to distant sites is the most harmful event during cancer progression, and the leading cause of cancer death. We have previously demonstrated that restoration of DLC1 tumor suppressor gene expression in the DLC1-negative Focus and 7703K human hepatocellular carcinoma (HCC) cell lines induced caspase-3 mediated apoptosis, reduced cell growth in vitro and tumorigenicity in vivo and diminished the ability to migrate through Matrigel, a property suggestive of metastatic potential in vivo. We now show that subcutaneous tumors developing after inoculation of Focus and 7703K cells into nude mice disseminate cells to liver and lung, and this process is markedly suppressed by restoration of DLC1 expression. Inhibition of tumor cell dissemination was associated with lower levels of RhoA activity, an increase in rounded cells and a reduction in actin stress fibers and focal adhesion molecules that are of critical importance in cancer cell invasion and metastasis. In addition, DLC1 down-regulated the expression of osteopontin and matrix metalloproteinase-9, which are highly up-regulated in most primary HCC with associated metastases. These observations implicate the DLC1 gene in suppression of HCC cell dissemination and identify novel cellular and genetic alterations that contribute to prevention of metastasis, a life-threatening event in cancer progression.
Figures
Figure 1.
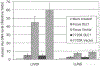
Detection of human tumor cells dissemination in liver and lung of athymic mice. The levels of human Alu DNA, representing dissemination of subcutaneously injected HCC cells, were quantitated by real-time PCR. Three- to 13-fold more human Alu DNA were detected in the lungs and livers of mice injected with 7703K/Vector and Focus/Vector cells than in tissues of mice injected with 7703K/DLC1 and Focus/DLC1 cells. There was no meaningful level of human Alu DNA in lungs and livers of mock injected mice.
Figure 2.
Expression of DLC1 inhibits RhoA activity. RhoA activity was two to three times lower in cells transduced with DLC1 (7703K/Ad-DLC1 and Focus/Ad-DLC1) than in cells transduced with the LacZ control (7703K/Ad-LacZ and Focus/Ad-LacZ). The data shown are the mean levels of RhoA activity ± SE from three independent experiments.
Figure 3.
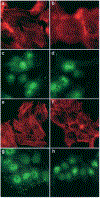
DLC1 expression affects organization and distribution of actin filaments and focal adhesions. Focus (b) and 7703K (f) cells expressing DLC1 exhibit fewer long actin stress fibers compared to their respective DLC1-negative controls (a and e). Similarly, DLC1-positive cells from both Focus (d) and 7703K (h) show a reduced number of vinculin-positive focal adhesion-like structures compared to their respective DLC1-negative controls (c and g).
Figure 4.
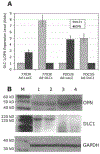
Reduced OPN expression in DLC1-transfected cells. (A) The levels of DLC1 and OPN mRNA in cells transiently transfected with Ad/DLC1 or the control vector were measured by real-time RT-PCR. A significant reduction of OPN gene expression was detected in Focus/DLC1 and 7703K/DLC1 cells compared to Focus/vector and 7703K/vector cells. (B) Western blotting demonstrated that OPN protein levels were lower in Focus/DLC1 and 7703K/DLC1 stably transfected cells than in control cells. M, molecular weight markers; lane 1, 7703K/DLC1; lane 2, Focus/DLC1; lane 3, 7703K/vector; and lane 4, Focus/vector. Blots were stained with antibodies against OPN (top panel), DLC1 (center panel) and GAPDH (bottom panel).
Figure 5.
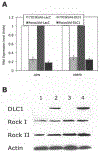
Comparative analysis of DLC1, OPN, MMP-9 and ROCK in DLC1 positive and negative HCC cells. (A) Down-regulation of OPN and MMP-9 expression in Focus/Ad-DLC1 and 7703K/Ad-DLC1 cells relative to Ad-LacZ transduced cells was quantitated by real-time PCR. (B) No alteration of ROCK protein was found in DLC1-transduced cell lines. Lane 1, 7703K/Ad-LacZ; lane 2, 7703K/Ad-DLC1; lane 3, Focus/Ad-LacZ; and lane 4, Focus/Ad-DLC1. Western blots were incubated with antibodies against DLC1, ROCK I, ROCK II and actin.
Similar articles
- DLC-1 suppresses non-small cell lung cancer growth and invasion by RhoGAP-dependent and independent mechanisms.
Healy KD, Hodgson L, Kim TY, Shutes A, Maddileti S, Juliano RL, Hahn KM, Harden TK, Bang YJ, Der CJ. Healy KD, et al. Mol Carcinog. 2008 May;47(5):326-37. doi: 10.1002/mc.20389. Mol Carcinog. 2008. PMID: 17932950 Free PMC article. - Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma.
Wong CM, Yam JW, Ching YP, Yau TO, Leung TH, Jin DY, Ng IO. Wong CM, et al. Cancer Res. 2005 Oct 1;65(19):8861-8. doi: 10.1158/0008-5472.CAN-05-1318. Cancer Res. 2005. PMID: 16204057 - Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma.
Wong CC, Wong CM, Ko FC, Chan LK, Ching YP, Yam JW, Ng IO. Wong CC, et al. PLoS One. 2008 Jul 23;3(7):e2779. doi: 10.1371/journal.pone.0002779. PLoS One. 2008. PMID: 18648664 Free PMC article. - Role of DLC1 tumor suppressor gene and MYC oncogene in pathogenesis of human hepatocellular carcinoma: potential prospects for combined targeted therapeutics (review).
Zimonjic DB, Popescu NC. Zimonjic DB, et al. Int J Oncol. 2012 Aug;41(2):393-406. doi: 10.3892/ijo.2012.1474. Epub 2012 May 10. Int J Oncol. 2012. PMID: 22580498 Free PMC article. Review. - Deleted in liver cancer-1 (DLC1): an emerging metastasis suppressor gene.
Popescu NC, Goodison S. Popescu NC, et al. Mol Diagn Ther. 2014 Jun;18(3):293-302. doi: 10.1007/s40291-014-0086-3. Mol Diagn Ther. 2014. PMID: 24519699 Free PMC article. Review.
Cited by
- Molecular alterations associated with breast cancer mortality.
Voeghtly LM, Mamula K, Campbell JL, Shriver CD, Ellsworth RE. Voeghtly LM, et al. PLoS One. 2012;7(10):e46814. doi: 10.1371/journal.pone.0046814. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056464 Free PMC article. - Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers.
Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Cao DX, et al. World J Gastroenterol. 2012 Aug 14;18(30):3923-30. doi: 10.3748/wjg.v18.i30.3923. World J Gastroenterol. 2012. PMID: 22912540 Free PMC article. Review. - Deleted in liver cancer 1 (DLC1) utilizes a novel binding site for Tensin2 PTB domain interaction and is required for tumor-suppressive function.
Chan LK, Ko FC, Ng IO, Yam JW. Chan LK, et al. PLoS One. 2009;4(5):e5572. doi: 10.1371/journal.pone.0005572. Epub 2009 May 15. PLoS One. 2009. PMID: 19440389 Free PMC article. - Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility.
Kim TY, Vigil D, Der CJ, Juliano RL. Kim TY, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):77-83. doi: 10.1007/s10555-008-9167-2. Cancer Metastasis Rev. 2009. PMID: 19221866 Free PMC article. Review. - Curcumin inhibits the growth of triple-negative breast cancer cells by silencing EZH2 and restoring DLC1 expression.
Zhou X, Jiao D, Dou M, Zhang W, Lv L, Chen J, Li L, Wang L, Han X. Zhou X, et al. J Cell Mol Med. 2020 Sep;24(18):10648-10662. doi: 10.1111/jcmm.15683. Epub 2020 Jul 28. J Cell Mol Med. 2020. PMID: 32725802 Free PMC article.
References
- Weinberg RA: Moving out: invasion and metastasis In: The Biology of Cancer. Garland Science, Taylor & Francis Group, LLC; New York, pp 587–655, 2007.
- Clark EA, Golub TR, Lander ES and Hynes RO: Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406: 532–535, 2000. - PubMed
- Nguyen DX and Massague J: Genetic determinants of cancer metastasis. Nat Rev Genet 8: 341–352, 2007. - PubMed
- Yuan BZ, Miller MJ, Keck CL, Zimonjic D, Thorgeirsson SS and Popescu NC: Cloning, characterization and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homogous to rat RhoGAP. Cancer Res 58: 2196–2199, 1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials