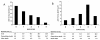Competing risk and heterogeneity of treatment effect in clinical trials - PubMed (original) (raw)
Editorial
Competing risk and heterogeneity of treatment effect in clinical trials
David M Kent et al. Trials. 2008.
Abstract
It has been demonstrated that patients enrolled in clinical trials frequently have a large degree of variation in their baseline risk for the outcome of interest. Thus, some have suggested that clinical trial results should routinely be stratified by outcome risk using risk models, since the summary results may otherwise be misleading. However, variation in competing risk is another dimension of risk heterogeneity that may also underlie treatment effect heterogeneity. Understanding the effects of competing risk heterogeneity may be especially important for pragmatic comparative effectiveness trials, which seek to include traditionally excluded patients, such as the elderly or complex patients with multiple comorbidities. Indeed, the observed effect of an intervention is dependent on the ratio of outcome risk to competing risk, and these risks - which may or may not be correlated - may vary considerably in patients enrolled in a trial. Further, the effects of competing risk on treatment effect heterogeneity can be amplified by even a small degree of treatment related harm. Stratification of trial results along both the competing and the outcome risk dimensions may be necessary if pragmatic comparative effectiveness trials are to provide the clinically useful information their advocates intend.
Figures
Figure 1
Relative and Absolute Benefits for Implantable Cardiac Defibrillators (ICDs) Stratified by Total Mortality Risk: These graphs show the relative (A) and absolute (B) benefit for ICDs assuming that the devices are 75% effective in preventing sudden cardiac death (SCD) but not at all effective in preventing pump failure. The risk ratio of SCD to pump failure death was empirically observed [30]. Note that both relative risk reduction decreases monotonically. Absolute risk reduction demonstrates a U-shaped benefit; benefit is low in low risk groups whose risk of SCD is low and in high risk groups who are suffer pump failure.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - Ablation: Atrial fibrillation: diagnosis and management: Network meta-analysis J2.
National Guideline Centre (UK). National Guideline Centre (UK). London: National Institute for Health and Care Excellence (NICE); 2021 Apr. London: National Institute for Health and Care Excellence (NICE); 2021 Apr. PMID: 34165931 Free Books & Documents. Review. - Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications.
Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Varadhan R, et al. Med Care. 2010 Jun;48(6 Suppl):S96-105. doi: 10.1097/MLR.0b013e3181d99107. Med Care. 2010. PMID: 20473207 Review. - Identifying treatment effect heterogeneity in clinical trials using subpopulations of events: STEPP.
Lazar AA, Bonetti M, Cole BF, Yip WK, Gelber RD. Lazar AA, et al. Clin Trials. 2016 Apr;13(2):169-79. doi: 10.1177/1740774515609106. Epub 2015 Oct 22. Clin Trials. 2016. PMID: 26493094 Free PMC article. Clinical Trial.
Cited by
- Caring for patients with kidney disease: shifting the paradigm from evidence-based medicine to patient-centered care.
O'Hare AM, Rodriguez RA, Bowling CB. O'Hare AM, et al. Nephrol Dial Transplant. 2016 Mar;31(3):368-75. doi: 10.1093/ndt/gfv003. Epub 2015 Jan 29. Nephrol Dial Transplant. 2016. PMID: 25637639 Free PMC article. Review. - How to integrate multiple comorbidities in guideline development: article 10 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.
Fabbri LM, Boyd C, Boschetto P, Rabe KF, Buist AS, Yawn B, Leff B, Kent DM, Schünemann HJ; ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development. Fabbri LM, et al. Proc Am Thorac Soc. 2012 Dec;9(5):274-81. doi: 10.1513/pats.201208-063ST. Proc Am Thorac Soc. 2012. PMID: 23256171 Free PMC article. Review. - Assessing Heterogeneity of Treatment Effect in Real-World Data.
Segal JB, Varadhan R, Groenwold RHH, Li X, Nomura K, Kaplan S, Ardeshirrouhanifard S, Heyward J, Nyberg F, Burcu M. Segal JB, et al. Ann Intern Med. 2023 Apr;176(4):536-544. doi: 10.7326/M22-1510. Epub 2023 Mar 21. Ann Intern Med. 2023. PMID: 36940440 Free PMC article. - A standardized framework for risk-based assessment of treatment effect heterogeneity in observational healthcare databases.
Rekkas A, van Klaveren D, Ryan PB, Steyerberg EW, Kent DM, Rijnbeek PR. Rekkas A, et al. NPJ Digit Med. 2023 Mar 30;6(1):58. doi: 10.1038/s41746-023-00794-y. NPJ Digit Med. 2023. PMID: 36991144 Free PMC article. - Clinical Trials in Veterinary Medicine: A New Era Brings New Challenges.
Oyama MA, Ellenberg SS, Shaw PA. Oyama MA, et al. J Vet Intern Med. 2017 Jul;31(4):970-978. doi: 10.1111/jvim.14744. Epub 2017 May 30. J Vet Intern Med. 2017. PMID: 28557000 Free PMC article.
References
- Cameron HJ, Williams BO. Clinical trials in the elderly should we do more? Drugs Aging. 1996;9:307–310. - PubMed
Publication types
LinkOut - more resources
Full Text Sources