Two-dimensional infrared spectra of isotopically diluted amyloid fibrils from Abeta40 - PubMed (original) (raw)
Two-dimensional infrared spectra of isotopically diluted amyloid fibrils from Abeta40
Yung Sam Kim et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The 2D IR spectra of the amide-I vibrations of amyloid fibrils from Abeta40 were obtained. The matured fibrils formed from strands having isotopic substitution by (13)C (18)O at Gly-38, Gly-33, Gly-29, or Ala-21 show vibrational exciton spectra having reduced dimensionality. Indeed, linear chain excitons of amide units are seen, for which the interamide vibrational coupling is measured in fibrils grown from 50% and 5% mixtures of labeled and unlabeled strands. The data prove that the 1D excitons are formed from parallel in-register sheets. The coupling constants show that for each of the indicated residues the amide carbonyls in the chains are separated by 0.5 +/- 0.05 nm. The isotope replacement of Gly-25 does not reveal linear excitons, consistent with the region of the strand having a different structure distribution. The vibrational frequencies of the amide-I modes, freed from effects of amide vibrational excitation exchange by 5% dilution experiments, point to there being a component of an electric field along the fibril axis that increases through the sequence Gly-38, Gly-33, Gly-29. The field is dominated by side chains of neighboring residues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
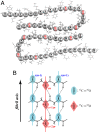
Fig. 1.
Diagrams of Aβ40 fibrils. (A) A cross section of two laterally displaced molecular layers of the Aβ40 fibril according to Petkova et al. (6). Residues 9–40 are shown. (B) Idealized structure of a portion of the parallel β-sheet. The strands are indicated by s, and the residue number in a given strand is indicated by n. The asterisk denotes the carbonyl group of a 13C 18O doubly labeled amide group. Dotted lines represent hydrogen bonds.
18O doubly labeled amide group. Dotted lines represent hydrogen bonds.
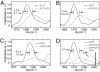
Fig. 2.
Linear and 2D IR spectra of Aβ40 doubly labeled with 13C 18O at Gly-38 (G38*) and a 1:1 mixture of G38* and unlabeled Aβ40 (G38*/G38) at different maturation times. (A–C) Linear IR spectra and 2D IR spectra at T = 0 of G38* at maturation times of 6 days (A), 12 days (B), and 19 days (C). (D–F) Linear IR spectra and 2D IR spectra at T = 0 of G38*/G38 at maturation times of 6 days (D), 12 days (E), and 19 days (F). The Inset in each 2D spectrum is an enlarged and four-times-intensified view of the area enclosed by the outlined region. The dotted circles in the linear spectra of A–C highlight the isotope-labeled amide-I transition regions. The magnitude of each 2D spectrum was scaled to have the same difference of maximum and minimum values.
18O at Gly-38 (G38*) and a 1:1 mixture of G38* and unlabeled Aβ40 (G38*/G38) at different maturation times. (A–C) Linear IR spectra and 2D IR spectra at T = 0 of G38* at maturation times of 6 days (A), 12 days (B), and 19 days (C). (D–F) Linear IR spectra and 2D IR spectra at T = 0 of G38*/G38 at maturation times of 6 days (D), 12 days (E), and 19 days (F). The Inset in each 2D spectrum is an enlarged and four-times-intensified view of the area enclosed by the outlined region. The dotted circles in the linear spectra of A–C highlight the isotope-labeled amide-I transition regions. The magnitude of each 2D spectrum was scaled to have the same difference of maximum and minimum values.
Fig. 3.
Experimental and simulated traces of the Aβ40 fibril 2D IR spectra at T = 0. (A–C) Traces of the 2D IR signal along a line ωt = ωτ + 2 cm−1 for A21* (solid) and A21*/A21 (dotted) (A), G33* (solid) and G33*/G33 (dotted) (B), and G38* (solid) and G38*/G38 (dotted) (C). (D) Simulated traces of the 2D IR spectra of fibrils formed from a 100% (solid), 50% (dotted), and 5% (dashed) G33-labeled peptide. The thick vertical line in D represents the frequency νo (defined in the text) of the isotope-labeled amide-I transition in the absence of any coupling. The coupling constant for the simulation of G33 (D) is α = −9.5 cm−1.
Fig. 4.
2D IR spectra of fibrils of G25* (13C 18O labeled at Gly-25) (A) and G29* (13C
18O labeled at Gly-25) (A) and G29* (13C 18O labeled at Gly-29) (B) at T = 0 after 70 days of maturation. In each spectrum the white area corresponds to a flat top off-scale signal. The contours begin at 25% of the peak signals. The Insets are enlarged and 10-times-intensified (A) and two-times-intensified (B) views of the marked areas.
18O labeled at Gly-29) (B) at T = 0 after 70 days of maturation. In each spectrum the white area corresponds to a flat top off-scale signal. The contours begin at 25% of the peak signals. The Insets are enlarged and 10-times-intensified (A) and two-times-intensified (B) views of the marked areas.
Similar articles
- 2D IR provides evidence for mobile water molecules in beta-amyloid fibrils.
Kim YS, Liu L, Axelsen PH, Hochstrasser RM. Kim YS, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17751-6. doi: 10.1073/pnas.0909888106. Epub 2009 Oct 8. Proc Natl Acad Sci U S A. 2009. PMID: 19815514 Free PMC article. - Insight into the internal structure of amyloid-β oligomers by isotope-edited Fourier transform infrared spectroscopy.
Baronio CM , Baldassarre M , Barth A . Baronio CM , et al. Phys Chem Chem Phys. 2019 Apr 17;21(16):8587-8597. doi: 10.1039/c9cp00717b. Phys Chem Chem Phys. 2019. PMID: 30964131 - Parallel β-sheet vibrational couplings revealed by 2D IR spectroscopy of an isotopically labeled macrocycle: quantitative benchmark for the interpretation of amyloid and protein infrared spectra.
Woys AM, Almeida AM, Wang L, Chiu CC, McGovern M, de Pablo JJ, Skinner JL, Gellman SH, Zanni MT. Woys AM, et al. J Am Chem Soc. 2012 Nov 21;134(46):19118-28. doi: 10.1021/ja3074962. Epub 2012 Nov 9. J Am Chem Soc. 2012. PMID: 23113791 Free PMC article. - Amide I two-dimensional infrared spectroscopy of proteins.
Ganim Z, Chung HS, Smith AW, Deflores LP, Jones KC, Tokmakoff A. Ganim Z, et al. Acc Chem Res. 2008 Mar;41(3):432-41. doi: 10.1021/ar700188n. Epub 2008 Feb 21. Acc Chem Res. 2008. PMID: 18288813 Review. - Coherent multidimensional vibrational spectroscopy of biomolecules: concepts, simulations, and challenges.
Zhuang W, Hayashi T, Mukamel S. Zhuang W, et al. Angew Chem Int Ed Engl. 2009;48(21):3750-81. doi: 10.1002/anie.200802644. Angew Chem Int Ed Engl. 2009. PMID: 19415637 Free PMC article. Review.
Cited by
- Discriminating early stage A{beta}42 monomer structures using chirality-induced 2DIR spectroscopy in a simulation study.
Zhuang W, Sgourakis NG, Li Z, Garcia AE, Mukamel S. Zhuang W, et al. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15687-92. doi: 10.1073/pnas.1002131107. Epub 2010 Aug 23. Proc Natl Acad Sci U S A. 2010. PMID: 20798063 Free PMC article. - Surface effects mediate self-assembly of amyloid-β peptides.
Lin YC, Petersson EJ, Fakhraai Z. Lin YC, et al. ACS Nano. 2014 Oct 28;8(10):10178-86. doi: 10.1021/nn5031669. Epub 2014 Sep 24. ACS Nano. 2014. PMID: 25229233 Free PMC article. - Effect of dehydration on the aggregation kinetics of two amyloid peptides.
Mukherjee S, Chowdhury P, Gai F. Mukherjee S, et al. J Phys Chem B. 2009 Jan 15;113(2):531-5. doi: 10.1021/jp809817s. J Phys Chem B. 2009. PMID: 19132862 Free PMC article. - Coherent multidimensional optical spectroscopy of excitons in molecular aggregates; quasiparticle versus supermolecule perspectives.
Abramavicius D, Palmieri B, Voronine DV, Sanda F, Mukamel S. Abramavicius D, et al. Chem Rev. 2009 Jun;109(6):2350-408. doi: 10.1021/cr800268n. Chem Rev. 2009. PMID: 19432416 Free PMC article. Review. No abstract available. - An alternative structural isoform in amyloid-like aggregates formed from thermally denatured human γD-crystallin.
Moran SD, Zhang TO, Zanni MT. Moran SD, et al. Protein Sci. 2014 Mar;23(3):321-31. doi: 10.1002/pro.2422. Epub 2014 Feb 4. Protein Sci. 2014. PMID: 24415662 Free PMC article.
References
- Eanes ED, Glenner GG. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968;16:673–677. - PubMed
- Sunde M, et al. Common core structure of amyloid fibrils by synchrotron x-ray diffraction. J Mol Biol. 1997;273:729–739. - PubMed
- Torok M, et al. Structural and dynamic features of Alzheimer's Aβ peptide in amyloid fibrils studied by site-directed spin labeling. J Biol Chem. 2002;277:40810–40815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM076201/GM/NIGMS NIH HHS/United States
- R37 GM012592/GM/NIGMS NIH HHS/United States
- P01RR01348/RR/NCRR NIH HHS/United States
- GM12592/GM/NIGMS NIH HHS/United States
- P41 RR001348/RR/NCRR NIH HHS/United States
- R01 GM012592/GM/NIGMS NIH HHS/United States
- GM 48310/GM/NIGMS NIH HHS/United States
- GM76201/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases