Reprogramming of pancreatic beta cells into induced pluripotent stem cells - PubMed (original) (raw)
Reprogramming of pancreatic beta cells into induced pluripotent stem cells
Matthias Stadtfeld et al. Curr Biol. 2008.
Abstract
Induced pluripotent stem (iPS) cells have been derived from fibroblast, stomach, and liver cultures at extremely low frequencies by ectopic expression of the transcription factors Oct4, Sox2, c-myc, and Klf4, a process coined direct or in vitro reprogramming [1-8]. iPS cells are molecularly and functionally highly similar to embryonic stem cells (ESCs), including their ability to contribute to all tissues as well as the germline in mice. The heterogeneity of the starting cell populations and the low efficiency of reprogramming suggested that a rare cell type, such as an adult stem cell, might be the cell of origin for iPS cells and that differentiated cells are refractory to reprogramming. Here, we used inducible lentiviruses [9] to express Oct4, Sox2, c-myc, and Klf4 in pancreatic beta cells to assess whether a defined terminally differentiated cell type remains amenable to reprogramming. Genetically marked beta cells gave rise to iPS cells that expressed pluripotency markers, formed teratomas, and contributed to cell types of all germ layers in chimeric animals. Our results provide genetic proof that terminally differentiated cells can be reprogrammed into pluripotent cells, suggesting that in vitro reprogramming is not restricted to certain cell types or differentiation stages.
Figures
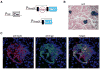
Figure 1. Culture and Viral Infection of Pancreatic Islets
(A–E) Bright-field (upper panel) and fluorescence (lower panel) images of a representative pancreatic islet isolated from Pdx1-GFP mice and imaged after the indicated culture periods. Note that most cells in the islet remain GFP+ and that islet cells stop expanding after one to two cell divisions. Rare GFP− fibroblast-like cells present in the cultures are indicated by arrows in (D) and (E). (F and G) Images of tail-tip fibroblasts (F) and islet cells from Pdx1-GFP mice (G) infected with a lentivirus constitutively expressing the red-fluorescent protein tdTomato.
Figure 2. Characterization of RIP-Cre/lacZ Pancreas
(A) Scheme illustrating β cell-specific activation of lacZ expression in RIP-Cre/lacZ mice. (B) Frozen pancreas sections from a RIP-Cre/lacZ mouse after Xgal staining shows islet specific labeling. (C) Immunofluorescence staining of a RIP-Cre/lacZ islet section with antibodies against insulin (red) and β-galactosidase (green) demonstrates exclusive expression of β-galactosidase in insulin-positive β cells. The position of a rare β cell not expressing β-galactosidase is highlighted by white arrows.
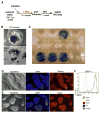
Figure 3. Generation of β iPS Cells
(A) Experimental outline. (B) Bright-field images showing two typical iPS colonies obtained after lentiviral infection of islet cultures. (C) Xgal staining of iPS lines derived from islets shows that 4 of the 12 depicted lines express β-galactosidase from the ROSA26 promoter. (D and E) Bright-field and fluorescent images of β iPS cells stained for the pluripotency markers Nanog (D) and Sox2 (E). Nuclei were counter-stained with DAPI. (F) FACS histograms of three β iPS cell lines and an ESC line stained for SSEA-1 (colored lines) or with an isotype control (gray line).
Figure 4. Developmental Potential of β iPS Cells
(A and B) Teratomas derived from β iPS (top image) as well as from fibroblast-derived iPS cells without a lacZ transgene (bottom image) were used for whole-mount Xgal staining. The light-blue staining seen in the control teratoma is due to weak background β-galactosidase activity. (C–E) Histological sections of teratomas derived from β iPS cells subjected to H&E staining (without Xgal staining) show muscle and glandular cells (C) and keratinized epithelium (D) as well as cartilagous tissue. (F) Images of newborn wild-type (left) and chimeric mice derived after blastocyst injection of β iPS. The tail of the chimeric mouse was used for establishing fibroblast cultures. (G–J) Images of Xgal-stained sections from a β iPS-derived newborn chimera show both Xgal+ (iPS-derived, black arrowheads) and Xgal− (blastocyst-derived, white arrowheads) cells in the vasculature (G), the bronchiolar epithelium of the lung (H), the cartilage of the rib (I), and neuronal tissue in the brain (J). Insets (red frames) show close-ups of chimeric areas.
Similar articles
- A Versatile In Vivo System to Study Myc in Cell Reprogramming.
Senís E, Mosteiro L, Grimm D, Abad M. Senís E, et al. Methods Mol Biol. 2021;2318:267-279. doi: 10.1007/978-1-0716-1476-1_14. Methods Mol Biol. 2021. PMID: 34019296 - Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors.
Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M, Schöler HR. Kim JB, et al. Nature. 2008 Jul 31;454(7204):646-50. doi: 10.1038/nature07061. Epub 2008 Jun 29. Nature. 2008. PMID: 18594515 - Emerging methods for preparing iPS cells.
Miyazaki S, Yamamoto H, Miyoshi N, Takahashi H, Suzuki Y, Haraguchi N, Ishii H, Doki Y, Mori M. Miyazaki S, et al. Jpn J Clin Oncol. 2012 Sep;42(9):773-9. doi: 10.1093/jjco/hys108. Epub 2012 Jul 23. Jpn J Clin Oncol. 2012. PMID: 22826352 Review. - [Induced pluripotent stem cells].
Shevchenko AI, Medvedev SP, Mazurok NA, Zakiian SM. Shevchenko AI, et al. Genetika. 2009 Feb;45(2):160-8. Genetika. 2009. PMID: 19334609 Review. Russian.
Cited by
- Induced pluripotent stem cells and their use in cardiac and neural regenerative medicine.
Skalova S, Svadlakova T, Shaikh Qureshi WM, Dev K, Mokry J. Skalova S, et al. Int J Mol Sci. 2015 Feb 13;16(2):4043-67. doi: 10.3390/ijms16024043. Int J Mol Sci. 2015. PMID: 25689424 Free PMC article. Review. - Spermatogonial stem cells, in vivo transdifferentiation and human regenerative medicine.
Simon L, Hess RA, Cooke PS. Simon L, et al. Expert Opin Biol Ther. 2010 Apr;10(4):519-30. doi: 10.1517/14712591003614731. Expert Opin Biol Ther. 2010. PMID: 20146635 Free PMC article. Review. - Nuclear receptor regulation of stemness and stem cell differentiation.
Jeong Y, Mangelsdorf DJ. Jeong Y, et al. Exp Mol Med. 2009 Aug 31;41(8):525-37. doi: 10.3858/emm.2009.41.8.091. Exp Mol Med. 2009. PMID: 19696553 Free PMC article. Review. - Inducible pluripotent stem cells: not quite ready for prime time?
Robbins RD, Prasain N, Maier BF, Yoder MC, Mirmira RG. Robbins RD, et al. Curr Opin Organ Transplant. 2010 Feb;15(1):61-7. doi: 10.1097/MOT.0b013e3283337196. Curr Opin Organ Transplant. 2010. PMID: 19855280 Free PMC article. Review. - Generation of Human Induced Pluripotent Stem Cells Using a Defined, Feeder-Free Reprogramming System.
Park S, Mostoslavsky G. Park S, et al. Curr Protoc Stem Cell Biol. 2018 May;45(1):e48. doi: 10.1002/cpsc.48. Epub 2018 May 4. Curr Protoc Stem Cell Biol. 2018. PMID: 30040234 Free PMC article.
References
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, et al. Directly reprogrammed fibroblasts show global epigenetic reprogramming and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. - PubMed
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources