Immunoparalysis and adverse outcomes from critical illness - PubMed (original) (raw)
Review
Immunoparalysis and adverse outcomes from critical illness
W Joshua Frazier et al. Pediatr Clin North Am. 2008 Jun.
Abstract
Proper immunologic balance between pro- and anti-inflammatory forces is necessary for recovery from critical illness. Persistence of a marked compensatory anti-inflammatory innate immune response after an insult is termed immunoparalysis. Critically ill patients demonstrating prolonged, severe reductions in monocyte HLA-DR expression or ex vivo tumor necrosis factor alpha production are at high risk for nosocomial infection and death. Reversal of immunoparalysis can be accomplished through the administration of immunostimulatory agents or tapering of exogenous immunosuppression. Evidence suggests that this may be associated with improved clinical outcomes. Immune-monitoring protocols are needed to identify patients who may benefit from immunomodulatory trials.
Figures
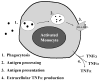
Figure 1. Functions of the activated monocyte
A normal monocyte, upon activation, is capable of ingesting pathogens; effecting intracellular killing and processing the foreign proteins into antigenic peptides; presenting these peptides on its cell surface in conjunction with HLA-DR molecules; and producing proinflammatory cytokines such as TNFα. An immunoparalyzed monocyte demonstrates reduced HLA-DR expression and impaired TNFα production capacity.
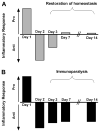
Figure 2. Immunologic homeostasis vs. immunoparalysis
A transient compensatory anti-inflammatory response typically follows a proinflammatory insult, but the immunologic balance between pro- and anti-inflammatory forces should be restored within a few days (A). If the anti-inflammatory response is severe and prolonged it is termed immunoparalysis (B).
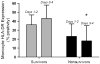
Figure 3. Prolonged suppression of monocyte HLA-DR expression predicts outcome
In 86 adult patients with septic shock, monocyte HLA-DR expression was suppressed on Days 1 – 2 in all patients, but to a greater degree in nonsurvivors. By Days 3 – 4, survivors had begun to recover HLA-DR expression whereas it remained low in nonsurvivors. *p < 0.001 vs. survivors, Mann – Whitney test. Data represent median (25th – 75th %). Data from Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. Aug 2006;32(8):1175–1183.
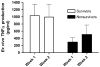
Figure 4. The ex vivo LPS-induced TNFα response is lower over time in nonsurvivors of pediatric MODS
Thirty children with MODS underwent serial measurement of their whole blood ex vivo LPS-induced TNFα response in the first and second weeks of illness. Nonsurvivors demonstrated a reduced capacity to produce TNFα when stimulated ex vivo over time compared to survivors (p = 0.017; 2-way ANOVA on log transformed data). Data represent mean (standard error). Data from Hall MW, Gavrilin MA, Knatz, NL et al. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatric Research. Nov 2007; 62(5):597–603.
Similar articles
- Inflammation and innate immune function in critical illness.
Muszynski JA, Thakkar R, Hall MW. Muszynski JA, et al. Curr Opin Pediatr. 2016 Jun;28(3):267-73. doi: 10.1097/MOP.0000000000000352. Curr Opin Pediatr. 2016. PMID: 27043087 Review. - Immunoparalysis in Pediatric Critical Care.
Hall MW, Greathouse KC, Thakkar RK, Sribnick EA, Muszynski JA. Hall MW, et al. Pediatr Clin North Am. 2017 Oct;64(5):1089-1102. doi: 10.1016/j.pcl.2017.06.008. Epub 2017 Aug 18. Pediatr Clin North Am. 2017. PMID: 28941537 Free PMC article. Review. - Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome.
Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA. Hall MW, et al. Intensive Care Med. 2011 Mar;37(3):525-32. doi: 10.1007/s00134-010-2088-x. Epub 2010 Dec 10. Intensive Care Med. 2011. PMID: 21153402 Free PMC article. Clinical Trial. - Critical Illness-Induced Immune Suppression: Current State of the Science.
Greathouse KC, Hall MW. Greathouse KC, et al. Am J Crit Care. 2016 Jan;25(1):85-92. doi: 10.4037/ajcc2016432. Am J Crit Care. 2016. PMID: 26724299 - Immunoparalysis in critically ill children.
Neves FL, Amaral MNGA, da Silva SFD, Silva IMM, Laranjeira PMDS, Pinto CRJ, Paiva AA, Dias ASDS, Coelho MLACV. Neves FL, et al. Immunology. 2023 Apr;168(4):597-609. doi: 10.1111/imm.13595. Epub 2022 Nov 14. Immunology. 2023. PMID: 36279244
Cited by
- Multiple MoS2 Transistors for Sensing Molecule Interaction Kinetics.
Nam H, Oh BR, Chen P, Chen M, Wi S, Wan W, Kurabayashi K, Liang X. Nam H, et al. Sci Rep. 2015 May 27;5:10546. doi: 10.1038/srep10546. Sci Rep. 2015. PMID: 26014289 Free PMC article. - An integrated microfluidic platform for in situ cellular cytokine secretion immunophenotyping.
Huang NT, Chen W, Oh BR, Cornell TT, Shanley TP, Fu J, Kurabayashi K. Huang NT, et al. Lab Chip. 2012 Oct 21;12(20):4093-101. doi: 10.1039/c2lc40619e. Lab Chip. 2012. PMID: 22892681 Free PMC article. - Prevention of nosocomial infections in critically ill patients with lactoferrin (PREVAIL study): study protocol for a randomized controlled trial.
Muscedere J, Maslove D, Boyd JG, O'Callaghan N, Lamontagne F, Reynolds S, Albert M, Hall R, McGolrick D, Jiang X, Day AG. Muscedere J, et al. Trials. 2016 Sep 29;17(1):474. doi: 10.1186/s13063-016-1590-z. Trials. 2016. PMID: 27681799 Free PMC article. - Thymoquinone modulates the expression of sepsis-related microRNAs in a CLP model.
Alkharfy KM, Ahmad A, Jan BL, Raish M, Rehman MU. Alkharfy KM, et al. Exp Ther Med. 2022 Jun;23(6):395. doi: 10.3892/etm.2022.11322. Epub 2022 Apr 14. Exp Ther Med. 2022. PMID: 35495595 Free PMC article. - Correlation of Plasma 25(OH)D3 and Vitamin D Binding Protein Levels with COVID-19 Severity and Outcome in Hospitalized Patients.
Alabdullatif W, Almnaizel A, Alhijji A, Alshathri A, Albarrag A, Bindayel I. Alabdullatif W, et al. Nutrients. 2023 Apr 10;15(8):1818. doi: 10.3390/nu15081818. Nutrients. 2023. PMID: 37111039 Free PMC article.
References
- Prophylactic intravenous administration of standard immune globulin as compared with core-lipopolysaccharide immune globulin in patients at high risk of postsurgical infection. The Intravenous Immunoglobulin Collaborative Study Group. N Engl J Med. 1992 Jul 23;327(4):234–240. - PubMed
- Calandra T, Glauser MP, Schellekens J, Verhoef J. Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind, randomized trial. J Infect Dis. 1988 Aug;158(2):312–319. - PubMed
- Bone RC, Balk RA, Fein AM, et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit Care Med. 1995 Jun;23(6):994–1006. - PubMed
- Greenman RL, Schein RM, Martin MA, et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. Jama. 1991 Aug 28;266(8):1097–1102. - PubMed
- McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med. 1994 Jul 1;121(1):1–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials