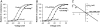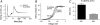Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids - PubMed (original) (raw)
Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids
Sara I Börjesson et al. Biophys J. 2008 Sep.
Abstract
Polyunsaturated fatty acids (PUFAs) have beneficial effects on epileptic seizures and cardiac arrhythmia. We report that omega-3 and omega-6 all-cis-PUFAs affected the voltage dependence of the Shaker K channel by shifting the conductance versus voltage and the gating charge versus voltage curves in negative direction along the voltage axis. Uncharged methyl esters of the PUFAs did not affect the voltage dependence, whereas changes of pH and charge mutations on the channel surface affected the size of the shifts. This suggests an electrostatic effect on the channel's voltage sensors. Monounsaturated and saturated fatty acids, as well as trans-PUFAs did not affect the voltage dependence. This suggests that fatty acid tails with two or more cis double bonds are required to place the negative carboxylate charge of the PUFA in a position to affect the channel's voltage dependence. We propose that charged lipophilic compounds could play a role in regulating neuronal excitability by electrostatically affecting the channel's voltage sensor. We believe this provides a new approach for pharmacological treatment that is voltage sensor pharmacology.
Figures
FIGURE 1
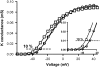
Procedure for measuring the shift of the G(V) curve. Data for control (○), 70 _μ_M eicosapentaenoic acid (□), and recovery (○) are shown together with Boltzmann raised to the power of n curves (continuous lines; Eq. 1). The area of interest for measurement is enlarged in the inset. The shift was measured at 10% of the maximum conductance.
FIGURE 2
The importance of the charge for shifting the G(V) curve. (A) 210 _μ_M DHA (□) shifted the control G(V) curve (○) in negative direction. The scaled DHA curve (▪) overlaps the control curve shifted −6.0 mV (•). Mean shift is −8.0 ± 0.7 mV (n = 7). (B) Addition of a methyl group to the carboxyl end abolished the shift (1.1 ± 0.8 mV, n = 5). Control (○), DHA-me (□). (C) The positively charged spider toxin GsMTx4 (□) shifted the control G(V) curve (○) in positive direction (+5.7 ± 0.3, n = 4). (D) A summary of the effects of DHA, DHA-me, and GsMTx4 on the channel's voltage dependence. Error bars indicate SE.
FIGURE 3
pH dependence of the DHA effects. (A) 70 _μ_M DHA at pH 9.0 increased the current at −40 mV 20-fold. (B) The corresponding G(V) curve was shifted −20.2 (mean shift −18.0 ± 1.4 mV, n = 9). Control (○), DHA (□). (C) DHA-me did not shift G(V) at pH 9.0. Control (○), DHA-me (□). (D) Shift data for DHA at pH 6.5 (•), pH 7.4 (○), pH 9.0 (□), and pH 10.0 (▪). Continuous lines show dose-response fits (Eq. 2) for pH 7.4 and 9.0. pH 7.4: Δ_V_max = −9.6 mV, _c_0.5 = 79 _μ_M; pH 9.0: Δ_V_max = −19.7 mV, _c_0.5 = 5.5 _μ_M. Error bars indicate SE. Small error bars are covered by symbols.
FIGURE 4
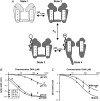
A simple four-state model describing the pH dependence of the FFA effects. (A) At low pH the ion channel conformation prevents FFA interaction (State 1). Increased pH induces conformational changes making FFA interaction possible (State 2). The FFA can interact with the channel in a protonated form (State 3) or a deprotonated form (State 4). The FFA can only change the channel's voltage dependence in State 4. (B) Global least-square fitted solutions for Eq. 3 shown as continuous lines. Δ_V_MAX = −20.5 mV, pKa,FFA = 7.4, _K_D = 3.5 _μ_M, and pKa,rec = 8.8. (C) The mutation line shows the predicted dose-response relationship from Eq. 3 for the triple mutation 419C-ET+/425K/451K at pH 7.4. Experimental data for the triple mutation are plotted as mean (Δ) ± SE. Small error bars are covered by symbols.
FIGURE 5
DHA affects the screening effect of a divalent metal ion. (A) Increased extracellular Mg2+ concentration (20 mM) shifted G(V) in positive direction by screening the negative surface charges. (B) DHA increased the screening effect (ΔΔ_V_Mg is 1.8 ± 0.3 mV, n = 10). (C) ΔΔ_V_Mg plotted versus DHA (□) and DHA-me (○) shifts for wt Shaker and DHA for the triple-positive mutant (▪) (data for 210 _μ_M). Error bars show SE. pH = 7.4. The continuous line shows the solution to the Grahame equation (Eq. 5).
FIGURE 6
Effect of DHA on the gating currents. (A) On and off-gating currents for control (black trace) and 70 _μ_M DHA (gray trace) at pH 9.0. DHA speeds up the on-gating current (extended timescale in inset) and slows down the off-gating current. The holding potential was −80 mV, the on-gating step to −20 mV, and the off-gating step to −80 mV. (B) 70 _μ_M DHA (□) at pH 9.0 shifted the control Q(V) curve (○) in negative direction (−6.3 ± 0.5 mV, n = 7). Dashed line shows control curve shifted −5.0 mV. (C) The shift of Q(V) is ∼1/3 of the G(V) shift.
FIGURE 7
Structure of fatty acids and fatty acid-like compounds used and G(V) shifts for respective compound. All data are for a compound concentration of 70 _μ_M at pH 7.4 (except for data denoted with † that are for compound concentrations of 210 _μ_M). n denotes the number of experiments, and ††, arachidonic acid with 1 _μ_M indomethacin. Error bars indicate SE.
Similar articles
- Polyunsaturated fatty acids are potent openers of human M-channels expressed in Xenopus laevis oocytes.
Liin SI, Karlsson U, Bentzen BH, Schmitt N, Elinder F. Liin SI, et al. Acta Physiol (Oxf). 2016 Sep;218(1):28-37. doi: 10.1111/apha.12663. Epub 2016 Mar 23. Acta Physiol (Oxf). 2016. PMID: 26914447 - Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: a putative mechanism of antiseizure action.
Xu XP, Erichsen D, Börjesson SI, Dahlin M, Amark P, Elinder F. Xu XP, et al. Epilepsy Res. 2008 Jul;80(1):57-66. doi: 10.1016/j.eplepsyres.2008.03.013. Epub 2008 May 2. Epilepsy Res. 2008. PMID: 18448313 - Subtype-specific responses of hKv7.4 and hKv7.5 channels to polyunsaturated fatty acids reveal an unconventional modulatory site and mechanism.
Frampton DJA, Choudhury K, Nikesjö J, Delemotte L, Liin SI. Frampton DJA, et al. Elife. 2022 Jun 1;11:e77672. doi: 10.7554/eLife.77672. Elife. 2022. PMID: 35642964 Free PMC article. - Emerging issues of connexin channels: biophysics fills the gap.
Harris AL. Harris AL. Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review. - Use of voltage clamp fluorimetry in understanding potassium channel gating: a review of Shaker fluorescence data.
Horne AJ, Fedida D. Horne AJ, et al. Can J Physiol Pharmacol. 2009 Jun;87(6):411-8. doi: 10.1139/y09-024. Can J Physiol Pharmacol. 2009. PMID: 19526034 Review.
Cited by
- Synergistic effect of docosahexaenoic acid on anticonvulsant activity of valproic acid and lamotrigine in animal seizure models.
Gavzan H, Sayyah M, Sardari S, Babapour V. Gavzan H, et al. Naunyn Schmiedebergs Arch Pharmacol. 2015 Oct;388(10):1029-38. doi: 10.1007/s00210-015-1135-0. Epub 2015 May 29. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 26018398 - Modulation of plant TPC channels by polyunsaturated fatty acids.
Gutla PV, Boccaccio A, De Angeli A, Gambale F, Carpaneto A. Gutla PV, et al. J Exp Bot. 2012 Oct;63(17):6187-97. doi: 10.1093/jxb/ers272. J Exp Bot. 2012. PMID: 23105130 Free PMC article. - Electronic polymers in lipid membranes.
Johansson PK, Jullesson D, Elfwing A, Liin SI, Musumeci C, Zeglio E, Elinder F, Solin N, Inganäs O. Johansson PK, et al. Sci Rep. 2015 Jun 10;5:11242. doi: 10.1038/srep11242. Sci Rep. 2015. PMID: 26059023 Free PMC article. - Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain.
Yang YM, Arsenault J, Bah A, Krzeminski M, Fekete A, Chao OY, Pacey LK, Wang A, Forman-Kay J, Hampson DR, Wang LY. Yang YM, et al. Mol Psychiatry. 2020 Sep;25(9):2017-2035. doi: 10.1038/s41380-018-0240-0. Epub 2018 Sep 17. Mol Psychiatry. 2020. PMID: 30224722 Free PMC article. - In Silico Screening Identification of Fatty Acids and Fatty Acid Derivatives with Antiseizure Activity: In Vitro and In Vivo Validation.
Barrionuevo EM, Peralta E, Manzur De Nardi A, Monat J, Fallico MJ, Llanos MA, Gavernet L, Mustafá ER, Martin P, Talevi A. Barrionuevo EM, et al. Pharmaceutics. 2024 Jul 27;16(8):996. doi: 10.3390/pharmaceutics16080996. Pharmaceutics. 2024. PMID: 39204342 Free PMC article.
References
- Lefevre, F., and N. Aronson. 2000. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics. 105:E46. - PubMed
- Leaf, A., Y. F. Xiao, J. X. Kang, and G. E. Billman. 2003. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacol. Ther. 98:355–377. - PubMed
- McKay, M. C., and F. W. Jennings. 2001. Linoleic acid both enhances activation and blocks Kv1.5 and Kv2.1 channels by two separate mechanisms. Am. J. Physiol. 281:1277–1284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources