Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by "helper" heterotrophic bacteria - PubMed (original) (raw)
Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by "helper" heterotrophic bacteria
J Jeffrey Morris et al. Appl Environ Microbiol. 2008 Jul.
Abstract
Axenic (pure) cultures of marine unicellular cyanobacteria of the Prochlorococcus genus grow efficiently only if the inoculation concentration is large; colonies form on semisolid medium at low efficiencies. In this work, we describe a novel method for growing Prochlorococcus colonies on semisolid agar that improves the level of recovery to approximately 100%. Prochlorococcus grows robustly at low cell concentrations, in liquid or on solid medium, when cocultured with marine heterotrophic bacteria. Once the Prochlorococcus cell concentration surpasses a critical threshold, the "helper" heterotrophs can be eliminated with antibiotics to produce axenic cultures. Our preliminary evidence suggests that one mechanism by which the heterotrophs help Prochlorococcus is the reduction of oxidative stress.
Figures
FIG. 1.
(A) Dilution series of Prochlorococcus strain MIT 9215 on a dilute lawn of approximately 1,000,000 cells of EZ55 on Pro99 agar medium lacking organic carbon. Numbers above the dilution spots indicate the dilution (_n_-fold) of the source culture of 3.5 × 108 cells of MIT 9215 ml−1; a 10-μl volume was spotted for each dilution. (B) Approximately 106 Prochlorococcus strain MIT 9215 cells were spread evenly onto agar. Cells of heterotrophic roseobacters Phaeobacter sp. strain Y4I and Sagittula stellata E-37 were then applied over the MIT 9215 cells as horizontal streaks (indicated by arrows). MIT 9215 cells (green) grew only where the heterotrophs were streaked.
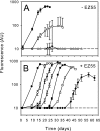
FIG. 2.
Growth of MIT 9215 in liquid Pro99 medium with or without helper heterotrophs. Dilutions of MIT 9215 were grown in the absence (A) or presence (B) of ∼5 × 105 CFU of EZ55 ml−1. Initial concentrations of MIT 9215 cells were 3.5 × 100 (⧫), 3.5 × 101 (□), 3.5 × 102 (▪), 3.5 × 103 (▵), 3.5 × 104 (▴), 3.5 × 105 (○), and 3.5 × 106 (•) cells ml−1. Error bars indicate one standard deviation of the mean for three replicate cultures. The dotted horizontal lines represent the limit of detection for chlorophyll-based fluorescence (10 arbitrary units [AU]); values below this limit were reported as 10 AU.
Similar articles
- Transcriptional response of Prochlorococcus to co-culture with a marine Alteromonas: differences between strains and the involvement of putative infochemicals.
Aharonovich D, Sher D. Aharonovich D, et al. ISME J. 2016 Dec;10(12):2892-2906. doi: 10.1038/ismej.2016.70. Epub 2016 Apr 29. ISME J. 2016. PMID: 27128996 Free PMC article. - Response of Prochlorococcus ecotypes to co-culture with diverse marine bacteria.
Sher D, Thompson JW, Kashtan N, Croal L, Chisholm SW. Sher D, et al. ISME J. 2011 Jul;5(7):1125-32. doi: 10.1038/ismej.2011.1. Epub 2011 Feb 17. ISME J. 2011. PMID: 21326334 Free PMC article. - Prochlorococcus Cells Rely on Microbial Interactions Rather than on Chlorotic Resting Stages To Survive Long-Term Nutrient Starvation.
Roth-Rosenberg D, Aharonovich D, Luzzatto-Knaan T, Vogts A, Zoccarato L, Eigemann F, Nago N, Grossart HP, Voss M, Sher D. Roth-Rosenberg D, et al. mBio. 2020 Aug 11;11(4):e01846-20. doi: 10.1128/mBio.01846-20. mBio. 2020. PMID: 32788385 Free PMC article. - Coevolution of marine phytoplankton and Alteromonas bacteria in response to pCO2 and coculture.
Lu Z, Entwistle E, Kuhl MD, Durrant AR, Barreto Filho MM, Goswami A, Morris JJ. Lu Z, et al. ISME J. 2025 Jan 2;19(1):wrae259. doi: 10.1093/ismejo/wrae259. ISME J. 2025. PMID: 39716385 Free PMC article. - Cross-protection from hydrogen peroxide by helper microbes: the impacts on the cyanobacterium Prochlorococcus and other beneficiaries in marine communities.
Zinser ER. Zinser ER. Environ Microbiol Rep. 2018 Aug;10(4):399-411. doi: 10.1111/1758-2229.12625. Epub 2018 Feb 23. Environ Microbiol Rep. 2018. PMID: 29411546 Review.
Cited by
- Microbial diversity of co-occurring heterotrophs in cultures of marine picocyanobacteria.
Kearney SM, Thomas E, Coe A, Chisholm SW. Kearney SM, et al. Environ Microbiome. 2021 Jan 6;16(1):1. doi: 10.1186/s40793-020-00370-x. Environ Microbiome. 2021. PMID: 33902739 Free PMC article. - Deciphering the prokaryotic community and metabolisms in South African deep-mine biofilms through antibody microarrays and graph theory.
Blanco Y, Rivas LA, García-Moyano A, Aguirre J, Cruz-Gil P, Palacín A, van Heerden E, Parro V. Blanco Y, et al. PLoS One. 2014 Dec 22;9(12):e114180. doi: 10.1371/journal.pone.0114180. eCollection 2014. PLoS One. 2014. PMID: 25531640 Free PMC article. - Relationship between abundance and specific activity of bacterioplankton in open ocean surface waters.
Hunt DE, Lin Y, Church MJ, Karl DM, Tringe SG, Izzo LK, Johnson ZI. Hunt DE, et al. Appl Environ Microbiol. 2013 Jan;79(1):177-84. doi: 10.1128/AEM.02155-12. Epub 2012 Oct 19. Appl Environ Microbiol. 2013. PMID: 23087033 Free PMC article. - Temperature Responses of Heterotrophic Bacteria in Co-culture With a Red Sea Synechococcus Strain.
Labban A, Palacio AS, García FC, Hadaidi G, Ansari MI, López-Urrutia Á, Alonso-Sáez L, Hong PY, Morán XAG. Labban A, et al. Front Microbiol. 2021 May 10;12:612732. doi: 10.3389/fmicb.2021.612732. eCollection 2021. Front Microbiol. 2021. PMID: 34040590 Free PMC article. - Effects of Bacterial Community Members on the Proteome of the Ammonia-Oxidizing Bacterium Nitrosomonas sp. Strain Is79.
Sedlacek CJ, Nielsen S, Greis KD, Haffey WD, Revsbech NP, Ticak T, Laanbroek HJ, Bollmann A. Sedlacek CJ, et al. Appl Environ Microbiol. 2016 Jul 15;82(15):4776-4788. doi: 10.1128/AEM.01171-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27235442 Free PMC article.
References
- Ahlgren, N. A., G. Rocap, and S. W. Chisholm. 2006. Measurement of Prochlorococcus ecotypes using real-time polymerase chain reaction reveals different abundances of genotypes with similar light physiologies. Environ. Microbiol. 8:441-454. - PubMed
- Alavi, M., T. Miller, K. Erlandson, R. Schneider, and R. Belas. 2001. Bacterial community associated with Pfiesteria-like dinoflagellate cultures. Environ. Microbiol. 3:380-396. - PubMed
- Cavender-Bares, K. K., S. L. Frankel, and S. W. Chisholm. 1998. A dual sheath flow cytometer for shipboard analyses of phytoplankton communities from oligotrophic seas. Limnol. Oceanogr. 43:1383-1388.
- Dusenberry, J. A., and S. L. Frankel. 1994. Increasing the sensitivity of a FACScan flow cytometer to study oceanic picoplankton. Limnol. Oceanogr. 39:206-209.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases