DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release - PubMed (original) (raw)
. 2008 Jun 2;181(5):791-801.
doi: 10.1083/jcb.200711021. Epub 2008 May 26.
Ya-Qin Feng, Chan-Juan Hao, Xiao-Li Guo, Xin He, Zhi-Yong Zhou, Ning Guo, Hong-Ping Huang, Wei Xiong, Hui Zheng, Pan-Li Zuo, Claire Xi Zhang, Wei Li, Zhuan Zhou
Affiliations
- PMID: 18504299
- PMCID: PMC2396815
- DOI: 10.1083/jcb.200711021
DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release
Xiao-Wei Chen et al. J Cell Biol. 2008.
Abstract
Schizophrenia is one of the most debilitating neuropsychiatric disorders, affecting 0.5-1.0% of the population worldwide. Its pathology, attributed to defects in synaptic transmission, remains elusive. The dystrobrevin-binding protein 1 (DTNBP1) gene, which encodes a coiled-coil protein, dysbindin, is a major susceptibility gene for schizophrenia. Our previous results have demonstrated that the sandy (sdy) mouse harbors a spontaneously occurring deletion in the DTNBP1 gene and expresses no dysbindin protein (Li, W., Q. Zhang, N. Oiso, E.K. Novak, R. Gautam, E.P. O'Brien, C.L. Tinsley, D.J. Blake, R.A. Spritz, N.G. Copeland, et al. 2003. Nat. Genet. 35:84-89). Here, using amperometry, whole-cell patch clamping, and electron microscopy techniques, we discovered specific defects in neurosecretion and vesicular morphology in neuroendocrine cells and hippocampal synapses at the single vesicle level in sdy mice. These defects include larger vesicle size, slower quantal vesicle release, lower release probability, and smaller total population of the readily releasable vesicle pool. These findings suggest that dysbindin functions to regulate exocytosis and vesicle biogenesis in endocrine cells and neurons. Our work also suggests a possible mechanism in the pathogenesis of schizophrenia at the synaptic level.
Figures
Figure 1.
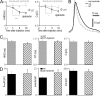
Dysbindin-deficient chromaffin cells display slow release kinetics and reduced depolarization-induced secretion. (A) A typical amperometric recording in response to a 20-s stimulus of 80 mM K+ in a WT chromaffin cell. Inset shows a micrograph of a microcarbon fiber electrode (black bar) attached to a chromaffin cell in an adrenal slice during amperometric recording. (B) Averaged traces showing the change in the shape of amperometric spikes in dysbindin-deficient (sdy) mice versus WT mice. (C, left) Three kinetics variables, i.e., HHD, RT, and Q, are defined. (rightmost three panels) Quantitative analyses of HHD, RT, and Q of amperometric spikes from WT (n = 166 events, 11 cells) and sdy mice (n = 131 events, 10 cells). (D) Examples of amperometric current traces (Iamp, top), integrated current signal (∫Iampdt), and membrane current traces (Im) evoked by a 2-s depolarizing pulse from −70 to +0 mV in a WT (gray) and a sdy (black) chromaffin cell. (inset) A micrograph of combined patch-clamp and amperometric recording in a chromaffin cell. (E) Histograms show the amount of secretion (integral of amperometric signal), the number of amperometric spikes, and the amplitude of the voltage-gated Ca2+ and Na+ currents of both cell types. ICa = 324.5 ± 34.6 pA (WT) and 339 ± 40 pA (sdy). INa = 3.0 ± 0.2 nA (WT) and 2.9 ± 0.2 nA (sdy). Data from 19 WT cells and 29 sdy cells are shown. *, P < 0.05; ***, P < 0.001. Error bars indicate the mean ± SEM. Bars, 10 μm.
Figure 2.
Microdialysis of purified dysbindin protein rescues the slow kinetics and reduced Q but not the probability and total amount of release in sdy mice. (A) Time course of the effects of intracellular dysbindin (black line) or heat-denatured dysbindin (gray line) on the kinetics of amperometric spikes and Q in sdy chromaffin cells. After establishing whole-cell mode, amperometric spikes were elicited every 5 min by a 2-s depolarization from −70 to 0 mV. The first points (−) represent data before dysbindin dialysis in sdy cells. The second, third, and fourth data points correspond to 1, 5, and 10 min after establishing whole-cell recording with 0.2 μg/μl full-length dysbindin or the denatured protein in the patch pipette. Note the appearance of fast kinetics of amperometric spikes at 5 min after dysbindin dialysis and the lack of difference between 5 and 10 min. In WT control, diamond data points indicate the mean values of amperometric spikes obtained at 5 and 10 min without dysbindin treatment. n = 201 spikes (−; 13 cells, 2 sdy mice), 116 spikes (1 min; 11 cells, 2 sdy mice), 94 spikes (5 min), 81 spikes (10 min), and 193 spikes (14 cells, 2 WT mice). (B) Averaged amperometric spikes obtained at 5 and 10 min after establishing whole-cell recording in untreated WT (gray) or sdy cells without (black) or with dysbindin addition (dotted line). Each line was averaged from 100 amperometric spikes, respectively. (C) In WT cells, histograms show the quantitative analyses of single spike properties (HHD, RT, and Q) without (gray) or with (striped) 5 and 10 min of intracellular dialysis of dysbindin. Data were obtained from the same adrenal slices from two WT littermates. n = 112 spikes (9 cells, WT) and 111 spikes (11 cells, WT + dysbindin). (D) Histograms show the amount of catecholamine secretion (∫Iampdt; left), the number of amperometric events per cell (middle), and calcium currents Ica (right) in sdy cells without (black; 13 cells, 2 sdy mice) or with 5 and 10 min dysbindin dialysis (striped; 11 cells, 2 sdy mice). Error bars indicate the mean ± SEM.
Figure 3.
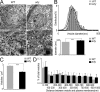
Electron micrographs show larger but fewer LDCVs in the absence of dysbindin. (A) Representative electron micrographs of adrenal sections from WT (left) and sdy (right) mice. LDCVs dispersed throughout the cytoplasm in both cell types. (insets) Single morphologically docked vesicles. Bars: (top) 2 μm; (bottom) 500 nm; (insets) 400 nm. (B) Distribution of vesicle diameters shows larger vesicles in sdy cells (black) than in WT (gray). Single Gaussian fittings of the distribution are superimposed on the bar graphs. Mean vesicle diameters were 214 ± 2 nm in the WT and 238 ± 2 nm in sdy (bottom). n = 973 (WT) and 1,095 vesicles (sdy). (C) Histogram shows that the lack of dysbindin (sdy) leads to a reduced number of LDCVs per μm2 as compared with WT cells. WT, 6.6 ± 0.2 vesicles per μm2 (21 cells from two WT mice); sdy, 4.9 ± 0.1 vesicles per μm2 (19 cells from three sdy mice). (D) Distribution of distance between vesicle membrane and plasma membrane. Vesicles located within 100 nm from the plasma membrane were defined as docked vesicles. ***, P < 0.001. Error bars indicate the mean ± SEM.
Figure 4.
Both miniature and evoked glutamate release in hippocampal CA1 pyramidal neurons are affected in the absence of dysbindin. (A) Representative mEPSC traces from WT and sdy hippocampal neurons in the presence of 10 μM bicuculline (Bic) and 1 μM tetrodotoxin (TTX). (B) Cumulative probability of mEPSC amplitude in WT (gray) and sdy (black) mice. (inset) The quantitative results of amplitude; no difference was found between WT and sdy mice. (C) Cumulative distribution of mEPSC frequency in WT (gray) and sdy (black) mice. Inset shows that the lack of dysbindin notably decreased the mean frequency of mEPSCs. (D) Histograms display quantitative analysis of HHD, RT, and charge of mEPSCs, showing that the lack of dysbindin slows the kinetics and increases the charge of single vesicle release. Data are from 238 spikes (7 cells, WT) and 112 spikes (6 cells, sdy). (E) Analysis of decay time constant (τ) was performed on averaged mEPSCs obtained from 50 individual events. The decay time was best fitted with a single exponential function. The decay of EPSCs in sdy neurons (black) was slower than that in WT (gray). (F) Averaged evoked EPSC waveforms of WT and sdy cells from 10 traces. (G) Comparison of the amplitude, RT, charge transfer, or decay time constant (τ) of evoked EPSCs between WT (gray) and sdy (black) cells. The lack of dysbindin resulted in reduced amplitude and slower decay time without affecting the amount of release as measured by the charge transfer. Data are from 17 cells (WT) and 16 cells (sdy). *, P < 0.05; ***, P < 0.001. Error bars indicate the mean ± SEM.
Figure 5.
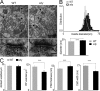
Dysbindin-deficient excitatory synapses have bigger but fewer vesicles in hippocampal CA1. (A; top) Electron micrographs depicting several asymmetrical synapses within CA1 in the ventral hippocampus. Double arrowheads indicate individual asymmetrical synapses. (bottom) Presynaptic terminals and adjacent dendritic spines. Arrowheads indicate docked vesicles; arrows mark the edges of the active zone/PSD complexes. Bars: (top) 500 nm; (bottom) 100 nm. (B) Distribution of vesicle sizes shows larger vesicles in sdy neurons (black) than in WT (gray). Gaussian fitting was performed. (bottom) The mean vesicle diameter of both cell types (WT, 44 ± 0.1 nm; sdy, 47 ± 0.1 nm; n = 1,015 vesicles from 121 WT synapses and 1,102 vesicles from 103 sdy synapses). (C) Comparison of four parameters, i.e., density of docked synaptic vesicles in active zone (WT, 20.5 ± 0.7 docked vesicles per μm; sdy, 20.2 ± 0.9 docked vesicles per μm), density of reserve pool (RP) vesicles in presynaptic terminal (WT, 110 ± 4 reserve pool vesicles per μm2; sdy, 86 ± 5 reserve pool vesicles per μm2), PSD thickness (WT, 44.2 ± 0.7 nm; sdy, 49 ± 1.0 nm), and synaptic cleft width (WT, 20.5 ± 0.2 nm; sdy, 16.0 ± 0.2 nm) in WT and sdy mice. ***, P < 0.001. Error bars indicate the mean ± SEM.
Figure 6.
Dysbindin deficiency produces a smaller RRP size of LDCVs in chromaffin cells. (A) Comparison of the kinetics of vesicle endocytosis in WT and sdy chromaffin cells. The cell was stimulated by a 200-ms depolarization from −70 to +10 mV. Depolarization-induced capacitance changes (ΔCm) were larger in WT cells than in sdy cells (P < 0.001). The kinetics of endocytosis in _sdy_ cells was similar to that in WT cells (P > 0.05). n = 7 (WT) and 6 (sdy). (B) Use of a dual-pulse protocol for estimating RRP size. Example of the capacitance responses (top) and Ca2+ currents (bottom) to a dual pulse. Two depolarizations with a 100-ms interval were applied. The depolarizing potentials were adjusted to give a similar amount of Ca2+ influx. (C) Comparison of the RRP size in WT (548.6 ± 92.3 fF) and sdy (287.0 ± 48.5 fF) cells, which were markedly different. n = 10 (WT) and 13 (sdy). (D) Comparison of the refilling kinetics of the RRP in WT and sdy cells. Normalized RRP size values were plotted versus the interpulse interval. Data points up to 10 s were fitted by a monoexponential curve. The interval between pulse pairs was ∼60 s. The time courses of RRP recovery were not significantly different. n = 10 (WT) and 13 (sdy). *, P < 0.05. Error bars indicate the mean ± SEM.
Figure 7.
Steady-state levels of synaptic proteins in sdy hippocampus and adrenal. (A) Immunoblotting analysis of hippocampus homogenates (30 μg) from WT and sdy mice showed no major changes in the expression levels of several known synaptic vesicle proteins. Signals were visualized with enhanced chemiluminescence. (B) Immunoblotting analysis of adrenal glands (80 μg) from WT and sdy animals. Dynamin I is not shown in the adrenal gland due to very weak bands under these experimental conditions, whereas VGLUT-1 is not expressed in adrenal glands. The immunoblots shown are representative of three independent experiments.
Similar articles
- Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia.
Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, Guo Y, Zhen XC, Li W. Feng YQ, et al. Schizophr Res. 2008 Dec;106(2-3):218-28. doi: 10.1016/j.schres.2008.07.018. Epub 2008 Sep 5. Schizophr Res. 2008. PMID: 18774265 - The sandy (sdy) mouse: a dysbindin-1 mutant relevant to schizophrenia research.
Talbot K. Talbot K. Prog Brain Res. 2009;179:87-94. doi: 10.1016/S0079-6123(09)17910-4. Epub 2009 Nov 20. Prog Brain Res. 2009. PMID: 20302821 Review. - Potential molecular mechanisms for decreased synaptic glutamate release in dysbindin-1 mutant mice.
Saggu S, Cannon TD, Jentsch JD, Lavin A. Saggu S, et al. Schizophr Res. 2013 May;146(1-3):254-63. doi: 10.1016/j.schres.2013.01.037. Epub 2013 Mar 6. Schizophr Res. 2013. PMID: 23473812 Free PMC article. - Loss of Dysbindin Implicates Synaptic Vesicle Replenishment Dysregulation as a Potential Pathogenic Mechanism in Schizophrenia.
Hu H, Wang X, Li C, Li Y, Hao J, Zhou Y, Yang X, Chen P, Shen X, Zhang S. Hu H, et al. Neuroscience. 2021 Jan 1;452:138-152. doi: 10.1016/j.neuroscience.2020.10.020. Epub 2020 Nov 10. Neuroscience. 2021. PMID: 33186610 - Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia?
Williams NM, O'Donovan MC, Owen MJ. Williams NM, et al. Schizophr Bull. 2005 Oct;31(4):800-5. doi: 10.1093/schbul/sbi061. Epub 2005 Sep 15. Schizophr Bull. 2005. PMID: 16166606 Review.
Cited by
- Overlapping Machinery in Lysosome-Related Organelle Trafficking: A Lesson from Rare Multisystem Disorders.
Banushi B, Simpson F. Banushi B, et al. Cells. 2022 Nov 21;11(22):3702. doi: 10.3390/cells11223702. Cells. 2022. PMID: 36429129 Free PMC article. Review. - Dysbindin-1 loss compromises NMDAR-dependent synaptic plasticity and contextual fear conditioning.
Glen WB Jr, Horowitz B, Carlson GC, Cannon TD, Talbot K, Jentsch JD, Lavin A. Glen WB Jr, et al. Hippocampus. 2014 Feb;24(2):204-13. doi: 10.1002/hipo.22215. Epub 2013 Nov 12. Hippocampus. 2014. PMID: 24446171 Free PMC article. - Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice.
Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Jentsch JD, et al. Neuropsychopharmacology. 2009 Nov;34(12):2601-8. doi: 10.1038/npp.2009.90. Epub 2009 Jul 29. Neuropsychopharmacology. 2009. PMID: 19641486 Free PMC article. - The BLOC-1 Subunit Pallidin Facilitates Activity-Dependent Synaptic Vesicle Recycling.
Chen X, Ma W, Zhang S, Paluch J, Guo W, Dickman DK. Chen X, et al. eNeuro. 2017 Feb 8;4(1):ENEURO.0335-16.2017. doi: 10.1523/ENEURO.0335-16.2017. eCollection 2017 Jan-Feb. eNeuro. 2017. PMID: 28317021 Free PMC article. - Loss of dysbindin-1 in mice impairs reward-based operant learning by increasing impulsive and compulsive behavior.
Carr GV, Jenkins KA, Weinberger DR, Papaleo F. Carr GV, et al. Behav Brain Res. 2013 Mar 15;241:173-84. doi: 10.1016/j.bbr.2012.12.021. Epub 2012 Dec 20. Behav Brain Res. 2013. PMID: 23261874 Free PMC article.
References
- Albillos, A., G. Dernick, H. Horstmann, W. Almers, G. Alvarez de Toledo, and M. Lindau. 1997. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 389:509–512. - PubMed
- Artalejo, C.R., M.E. Adams, and A.P. Fox. 1994. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 367:72–76. - PubMed
- Barbara, J.G., J.C. Poncer, R.A. McKinney, and K. Takeda. 1998. An adrenal slice preparation for the study of chromaffin cells and their cholinergic innervation. J. Neurosci. Methods. 80:181–189. - PubMed
- Benson, M.A., S.E. Newey, E. Martin-Rendon, R. Hawkes, and D.J. Blake. 2001. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J. Biol. Chem. 276:24232–24241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials