Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment - PubMed (original) (raw)
Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment
Julita A Teodorczyk-Injeyan et al. Chiropr Osteopat. 2008.
Abstract
Background: Increasing evidence supports somato-visceral effects of manual therapies. We have previously demonstrated that a single spinal manipulative treatment (SMT) accompanied by audible release has an inhibitory effect on the production of proinflammatory cytokines in asymptomatic subjects. The purpose of this study is to report on SMT-related changes in the production of the immunoregulatory cytokine interleukin 2 (IL-2) and to investigate whether such changes might differ with respect to the treatment approach related to the presence or absence of an audible release (joint cavitation).
Methods: Of 76 asymptomatic subjects, 29 received SMT with cavitation (SMT-C), 23 were treated with SMT without cavitation (SMT-NC) and 24 comprised the venipuncture control (VC) group. The SMT-C and SMT-NC subjects received a single, similar force high velocity low amplitude manipulation, in the upper thoracic spine. However, in SMT-NC subjects, positioning and line of drive were not conducive to cavitation. Blood and serum samples were obtained before and then at 20 and 120 min post-intervention. The production of IL-2 in peripheral blood mononuclear cell cultures was induced by activation for 48 hr with Staphylococcal protein A (SPA) and, in parallel preparations, with the combination of phorbol ester (TPA) and calcium ionophore. The levels of IL-2 in culture supernatants and serum were assessed by specific immunoassays.
Results: Compared with VC and their respective baselines, SPA-induced secretion of IL-2 increased significantly in cultures established from both SMT-C and SMT-NC subjects at 20 min post-intervention. At 2 hr post-treatment, significant elevation of IL-2 synthesis was still apparent in preparations from SMT-treated groups though it became somewhat attenuated in SMT-NC subjects. Conversely, IL-2 synthesis induced by TPA and calcium ionophore was unaltered by either type of SMT and was comparable to that in VC group at all time points. No significant alterations in serum-associated IL-2 levels were observed in any of the study groups.
Conclusion: The present study demonstrates that, the in vitro T lymphocyte response to a conventional mitogen (SPA), as measured by IL-2 synthesis, can become enhanced following SMT. Furthermore, within a period of time following the manipulative intervention, this effect may be independent of joint cavitation. Thus the results of this study suggest that, under certain physiological conditions, SMT might influence IL-2-regulated biological responses.
Figures
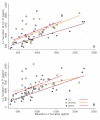
Figure 1
SPA-induced IL-2 secretion in cultures established 20 minutes (A) and 2 hours (B) post-intervention (PI). PBMC cultures were activated with SPA (10 μg/ml) at initiation and incubated at 37°C for 48 hr. The concentration of IL-2 (pg/ml) produced in vitro by cells from a subject at 20 min (A) and 2 hr (B) post intervention is plotted against the concentration of IL-2 produced at baseline by the same subject. Each point represents a subject for whom a complete set of data was available. Comparison of best fit lines revealed statistically significant differences between SMT-C (spinal manipulative therapy with cavitation, n = 26) and VC (venipuncture, n = 21) as well as SMT-NC (spinal manipulative therapy without cavitation, n = 23) and VC both for 20 min and 2 hr post intervention (see text for details).
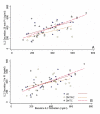
Figure 2
Phorbol ester (TPA)-induced IL-2 secretion in PBMC cultures established 20 minutes (A) and 2 hours (B) post-intervention (PI). PBMC cultures were activated with the combination of TPA (3 × 10-8 M) and calcium ionophore A23187 (200 ng/ml) at initiation and incubated at 37°C for 48 hr. IL-2 levels were determined in culture supernatants by specific ELISA. Each point represents the concentration of IL-2 (ng/ml) produced in vitro by cells from a subject at 20 min (A) and 2 hr (B) post intervention plotted against the concentration of IL-2 produced at baseline by the same subject. Each point represents a subject for whom a complete set of data was available. Comparison of best fit lines revealed no statistically significant differences between SMT-C (spinal manipulative therapy with cavitation, n = 22) and VC (venipuncture, n = 16) as well as SMT-NC (spinal manipulative therapy without cavitation, n = 17) and VC both for 20 min and 2 hr post intervention (see text for details).
Similar articles
- Interleukin 2-regulated in vitro antibody production following a single spinal manipulative treatment in normal subjects.
Teodorczyk-Injeyan JA, McGregor M, Ruegg R, Injeyan HS. Teodorczyk-Injeyan JA, et al. Chiropr Osteopat. 2010 Sep 8;18:26. doi: 10.1186/1746-1340-18-26. Chiropr Osteopat. 2010. PMID: 20825650 Free PMC article. - Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects.
Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Teodorczyk-Injeyan JA, et al. J Manipulative Physiol Ther. 2006 Jan;29(1):14-21. doi: 10.1016/j.jmpt.2005.10.002. J Manipulative Physiol Ther. 2006. PMID: 16396725 - Effects of spinal manipulative therapy on inflammatory mediators in patients with non-specific low back pain: a non-randomized controlled clinical trial.
Teodorczyk-Injeyan JA, Triano JJ, Gringmuth R, DeGraauw C, Chow A, Injeyan HS. Teodorczyk-Injeyan JA, et al. Chiropr Man Therap. 2021 Jan 8;29(1):3. doi: 10.1186/s12998-020-00357-y. Chiropr Man Therap. 2021. PMID: 33413508 Free PMC article. Clinical Trial. - Assessment of Studies Evaluating Spinal Manipulative Therapy and Infectious Disease and Immune System Outcomes: A Systematic Review.
Chow N, Hogg-Johnson S, Mior S, Cancelliere C, Injeyan S, Teodorczyk-Injeyan J, Cassidy JD, Taylor-Vaisey A, Côté P. Chow N, et al. JAMA Netw Open. 2021 Apr 1;4(4):e215493. doi: 10.1001/jamanetworkopen.2021.5493. JAMA Netw Open. 2021. PMID: 33847753 Free PMC article. - Peripheral response to cervical or thoracic spinal manual therapy: an evidence-based review with meta analysis.
Chu J, Allen DD, Pawlowsky S, Smoot B. Chu J, et al. J Man Manip Ther. 2014 Nov;22(4):220-9. doi: 10.1179/2042618613Y.0000000062. J Man Manip Ther. 2014. PMID: 25395830 Free PMC article. Review.
Cited by
- Short-term response of hip mobilizations and exercise in individuals with chronic low back pain: a case series.
Burns SA, Mintken PE, Austin GP, Cleland J. Burns SA, et al. J Man Manip Ther. 2011 May;19(2):100-7. doi: 10.1179/2042618610Y.0000000007. J Man Manip Ther. 2011. PMID: 22547920 Free PMC article. - The Physiological Role of Tumor Necrosis Factor in Human Immunity and Its Potential Implications in Spinal Manipulative Therapy: A Narrative Literature Review.
Zhang L, Yao CH. Zhang L, et al. J Chiropr Med. 2016 Sep;15(3):190-6. doi: 10.1016/j.jcm.2016.04.016. Epub 2016 May 26. J Chiropr Med. 2016. PMID: 27660595 Free PMC article. - The Effects Induced by Spinal Manipulative Therapy on the Immune and Endocrine Systems.
Colombi A, Testa M. Colombi A, et al. Medicina (Kaunas). 2019 Aug 7;55(8):448. doi: 10.3390/medicina55080448. Medicina (Kaunas). 2019. PMID: 31394861 Free PMC article. Review. - The Potential Mechanisms of High-Velocity, Low-Amplitude, Controlled Vertebral Thrusts on Neuroimmune Function: A Narrative Review.
Haavik H, Niazi IK, Kumari N, Amjad I, Duehr J, Holt K. Haavik H, et al. Medicina (Kaunas). 2021 May 27;57(6):536. doi: 10.3390/medicina57060536. Medicina (Kaunas). 2021. PMID: 34071880 Free PMC article. Review. - Quantification of cavitation and gapping of lumbar zygapophyseal joints during spinal manipulative therapy.
Cramer GD, Ross K, Raju PK, Cambron J, Cantu JA, Bora P, Dexheimer JM, McKinnis R, Habeck AR, Selby S, Pocius JD, Gregerson D. Cramer GD, et al. J Manipulative Physiol Ther. 2012 Oct;35(8):614-21. doi: 10.1016/j.jmpt.2012.06.007. Epub 2012 Aug 14. J Manipulative Physiol Ther. 2012. PMID: 22902194 Free PMC article. Clinical Trial.
References
- Sato A, Budgell B. Somatoautonomic reflexes. In: Haldeman S, editor. Principles and practice of chiropractic. New York, McGraw-Hill; 2005. pp. 301–314.
LinkOut - more resources
Full Text Sources