Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential - PubMed (original) (raw)
Review
Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential
Robert J Kolb et al. Front Biosci. 2008.
Abstract
The majority of different cell types in the human body have a cilium, a thin rod-like structure of uniquely arranged microtubules that are encapsulated by the surface plasma membrane. The cilium originates from a basal body, a mature centriole that has migrated and docked to the cell surface. The non-motile cilia are microtubule-based organelles that are generally considered sensory structures. The purpose of this review is to discuss the practicality of the ciliary hypothesis as a unifying concept for polycystic kidney disease and to review current literature in the field of cilium biology, as it relates to mechanosensation and planar cell polarity. The polycystins and fibrocystin localization at the cilium and other subcellular localizations are discussed, followed by a hypothetical model for the cilium's role in mechanosensing, planar cell polarity, and cystogenesis.
Figures
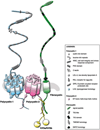
Figure 1
Mechanosensory protein complex. Among other subcellular localizations, it is thought that polycystin-1, polycystin-2 and fibrocystin form a mechanosensory complex protein in the cilium to sense fluid-shear stress. Polycystin-1 and polycystin-2 interact with each other at their COOH termini forming a polycystin complex. It is predicted that fibrocystin interacts with this complex through polycystin-2 via Kif as a possible adaptor protein.
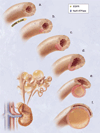
Figure 2
Hypothetical model of cystogenesis. The illustration depicts mechanosensory function of renal tubular epithelial cilium. a. Each cilium plays an important role to transmit extracellular information, such as urine flow, into the cell. This message may provide critical signals to the cell regarding the direction of cell division along the tubule. b. Insults, such as genetic disorder or random mutation, will result in abnormal ciliary function to sense fluid movement. c. The functional abnormality in ciliary sensing may result in loss of planar cell polarity. d. Direction of cell division becomes randomized, resulting in increasing tubular diameter rather than tubular elongation. e. Budding of a cyst from the renal tubule and abnormal localizations of epidermal growth factor receptor (EGFR) and Na+/K+ ATPase pump are typical characteristics of the autosomal dominant polycystic kidney. f. The cyst is eventually enlarged and isolated. Multiple cysts from the neighboring nephrons are illustrated on the bottom left corner.
Similar articles
- Cilium, centrosome and cell cycle regulation in polycystic kidney disease.
Lee K, Battini L, Gusella GL. Lee K, et al. Biochim Biophys Acta. 2011 Oct;1812(10):1263-71. doi: 10.1016/j.bbadis.2011.02.008. Epub 2011 Mar 2. Biochim Biophys Acta. 2011. PMID: 21376807 Free PMC article. Review. - Ciliopathies and the Kidney: A Review.
McConnachie DJ, Stow JL, Mallett AJ. McConnachie DJ, et al. Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review. - Putative roles of cilia in polycystic kidney disease.
Winyard P, Jenkins D. Winyard P, et al. Biochim Biophys Acta. 2011 Oct;1812(10):1256-62. doi: 10.1016/j.bbadis.2011.04.012. Epub 2011 May 8. Biochim Biophys Acta. 2011. PMID: 21586324 Review. - Cilia in cystic kidney and other diseases.
Pazour GJ, Quarmby L, Smith AO, Desai PB, Schmidts M. Pazour GJ, et al. Cell Signal. 2020 May;69:109519. doi: 10.1016/j.cellsig.2019.109519. Epub 2019 Dec 24. Cell Signal. 2020. PMID: 31881326 Free PMC article. Review. - Polycystic kidney disease: cell division without a c(l)ue?
Simons M, Walz G. Simons M, et al. Kidney Int. 2006 Sep;70(5):854-64. doi: 10.1038/sj.ki.5001534. Epub 2006 Jun 28. Kidney Int. 2006. PMID: 16816842 Review.
Cited by
- Glis3 is associated with primary cilia and Wwtr1/TAZ and implicated in polycystic kidney disease.
Kang HS, Beak JY, Kim YS, Herbert R, Jetten AM. Kang HS, et al. Mol Cell Biol. 2009 May;29(10):2556-69. doi: 10.1128/MCB.01620-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273592 Free PMC article. - Multiple urinary tract infections are associated with genotype and phenotype in adult polycystic kidney disease.
Eroglu E, Kocyigit I, Cetin M, Zararsiz G, Imamoglu H, Bayramov R, Tastan S, Sipahioglu MH, Tokgoz B, Oymak O. Eroglu E, et al. Clin Exp Nephrol. 2019 Oct;23(10):1188-1195. doi: 10.1007/s10157-019-01752-3. Epub 2019 Jun 5. Clin Exp Nephrol. 2019. PMID: 31165946 - GLIS1-3 transcription factors: critical roles in the regulation of multiple physiological processes and diseases.
Jetten AM. Jetten AM. Cell Mol Life Sci. 2018 Oct;75(19):3473-3494. doi: 10.1007/s00018-018-2841-9. Epub 2018 May 19. Cell Mol Life Sci. 2018. PMID: 29779043 Free PMC article. Review. - Hypertension in Autosomal Dominant Polycystic Kidney Disease: A Clinical and Basic Science Perspective.
Ratnam S, Nauli SM. Ratnam S, et al. Int J Nephrol Urol. 2010 Spring;2(2):294-308. Int J Nephrol Urol. 2010. PMID: 25364490 Free PMC article. - Primary cilia: highly sophisticated biological sensors.
Abou Alaiwi WA, Lo ST, Nauli SM. Abou Alaiwi WA, et al. Sensors (Basel). 2009;9(9):7003-20. doi: 10.3390/s90907003. Epub 2009 Sep 3. Sensors (Basel). 2009. PMID: 22423203 Free PMC article.
References
- Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–2536. - PubMed
- Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. - PubMed
- Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. - PubMed
- Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. - PubMed
- Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK080640/DK/NIDDK NIH HHS/United States
- HL084451/HL/NHLBI NIH HHS/United States
- R01 DK080640-01A1S1/DK/NIDDK NIH HHS/United States
- R01 DK080640-02/DK/NIDDK NIH HHS/United States
- R21 HL084451/HL/NHLBI NIH HHS/United States
- R01 DK080640-02S1/DK/NIDDK NIH HHS/United States
- R01 DK080640-01A1/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources