Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans - PubMed (original) (raw)
Review
Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans
Young-Kyung Bae et al. Front Biosci. 2008.
Abstract
In the free-living nematode Caenorhabditis elegans, cilia are found on the dendritic endings of sensory neurons. C. elegans cilia are classified as 'primary' or 'sensory' according to the '9+0' axonemal ultrastructure (nine doublet outer microtubules with no central microtubule pair) and lack of motility, characteristics of '9+2' cilia. The C. elegans ciliated nervous system allows the animal to perceive environmental stimuli and make appropriate developmental, physiological, and behavioral decisions. In vertebrates, the biological significance of primary cilia had been largely neglected. Recent findings have placed primary/sensory cilia in the center of cellular signaling and developmental processes. Studies using genetic model organisms such as C. elegans identified the link between ciliary dysfunction and human ciliopathies. Future studies in the worm will address important basic questions regarding ciliary development, morphogenesis, specialization, and signaling functions.
Figures
Figure 1
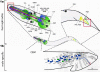
C. elegans ciliated sensory neurons. la. Ciliated sensory neurons are concentrated in the hermaphrodite head. This image is a lateral view from the left side. The core (non-sex specific) neuronal cell bodies are indicated with the names. The locations of ciliary endings are indicated as red box. ADL, ASG, ASI, ASK, ADF, ASE, ASH, and ASJ are amphid channel neurons. AWA, AWB, and AWC are amphid wing neurons. ADE, anterior deirid; AED, amphid finger; CEPD, cephalic dorsal; CEPV, cephalic ventral; IL1 and IL2, inner labial 1 and 2; OLL, outer labial lateral; OLQD, outer labial quadrant dorsal; OLQV, OLQ ventral; PDE, posterior deirid; PHA/PHB, phasmid A/B. This cartoon is adapted from the Wormbook chapter (Inglis et al.: The sensory cilia of C. elegans, (2007)) with permission, 1b. The male possesses additional ciliated CEMs neurons in the head (not shown). The rest of male-specific ciliated sensory neurons are located in the tail: ray RnA/RnB (n=1–9, blue), spicule (SPV and SPD, green), p.c.s (PCA, green), and hook HOA/HOB (not shown). In this figure, the positions of left nuclei are shown. R6B dendritic process is drawn (blue line) from the cell body to the ray tip, where the cilium resides (red box). CEM, cephalic male; PCA/PCB/PCC, postcloacal; PCso; postcloacal socket; PCh, postcloacal hypodermal; PCsh; postcloacal sheath; PLML, posterior lateral microtubule left, RnA/RnB, ray neuron A/B (n=1–9); Rnst; ray structural; SPC/SPD/SPV; spicule neurons; The schematics of other dendrites are similar. Reproduced from (6) Copyright (1980), with permission from Elsevier.
Figure 2
Ultrastiucture of C. elegans cilia. 2a. Cilia in the amphid sensillum exhibit a variety of morphologies. The rod-like channel cilia are found in ASE, ASG, ASH, ASI, ASJ, ASK, ADF, and ADL neurons. ADF and ADL possess two cilia each, while the other cells possess a single cilium. These cilia are exposed to the environment through the cuticle. The amphid wing neurons (AWA, AWB, AWC) have complex ciliary structures. The AFD neuron possesses multiple villi. The sheath and socket cell encapsulate the amphid channel cilia, whereas the sheath cell encloses the amphid wing (AWA/AWB/AWC) and AFD cilia. Cross section views are shown on the left (a–e). This is modified from (4), Copyright (1986), with permission from Elsevier. . 2b. The cephalic and outer labial sensillum. In the male, the CEM neuron is also located in the cephalic sensillum. 2c. The inner labial sensillium contains IL1 and IL2 neurons. IL2 is embedded within subcuticular structure, while IL1 is exposed to the exterior. 2b and 2c were modified from (1) Copyright (1975, Ward et al) with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. 2d. The phasmid sensillum in the tail. The PHA and PHB cilia extend in parallel to each other. Two phasmid socket cells (socket 1 and 2) surround the sensillum. 2e. The anterior deidrid ADE cilia. The image was provided by Sam Ward. 2f. Each ray sensillum contains a pair of cilia: RnA and RnB. RnA cilia are embedded whereas RnB (except R6B) cilia are exposed. The ray structural cell functions as both socket and sheath cell. 2g. The hook sensillum encloses HOA (embedded) and HOB (exposed). 2h. The spicule sensillum has SPV and SPD ciliated neurons, four syncytial socket cells, and two syncytial sheath cells. 2i. The postcloacal sensillum (p.c.s.) possesses one ciliated neuron (PCA), which ciliary tip ends within the cuticle. 2d and 2f–2i. Reproduced from (6) Copyright (1980), with permission from Elsevier.
Figure 3
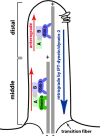
Intraflagellar transport (IFT) builds cilia. A simplified cartoon of IFT process in C. elegans amphid channel cilia. Two anterograde motors (OSM-3 and Kinesin-II) move IFT complexes on the microtubule doublets in the 4 um-long middle segment. In the 2 um-long distal segment, OSM-3 motor acts alone. The cytoplasmic dynein-2 (IFT-dynein) brings the IFT complex back to the base of the cilium. The six transmembrane protein in the ciliary membrane is a representative of sensory receptors in the cilium.
Figure 4
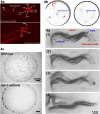
Assays for C. elegans ciliated neurons. 4a. Dye-filling assay. When worms are soaked in a lipophilic dye such as Dil, FITC, and DiO, certain ciliated sensory neurons fill with the dye. In this picture, Dil-filling in amphid and phasmid neurons are shown. This is adapted from Wormbook chapter (Shaham, Methods in Cell Biology (2005)) with permission. The original image is provided by Zeynep Altun,
. 4b. Chemotaxis. Worms are attracted to the Cl− gradient towards the center of the plate. The worm tracks from the three origins indicated by the red arrowheads (left panel). When water is placed in the center as control, worms do not exhibit chemoattraction behavior (right panel). These images are modified from Ward et al, (1973). 4c. Social feeding. The laboratory standard strain N2 is a solitary feeder (top panel), whereas a natural isolate CB4856 and npr-1 N2 mutants are social feeders (bottom panel). Social feeders also aggregate at the border of a bacterial lawn. These images are modified (61) Copyright (1998), with permission from Elsevier. 4d. Male Mating. (1) A male exhibits `response' behavior by starting a backward movement with his ventral tail on the hermaphrodite body. (2) While continuing backing, he turns at the end of the body. In search of the vulva, his tail scans along the hermaphrodite. (3, 4) At the vulva, he stops backing and adjusts the precise location by fine back and forth movements to insert spicules (vulva location and spicule insertion). These images are reproduced from (20) Copyright (1995), with permission from Elsevier.
Figure 5
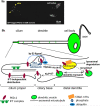
Trafficking of ciliary receptors, a. A GFP-tagged PKD-2 localizes to ciliary endings and cell bodies of the male-specific CEM head neurons. Dendritic vesicles are not evident in this image due to the low exposure setting, b. A working model for PKD-2 trafficking at the ciliary region. PKD-2 containing vesicles are transported through the dendrite. At the ciliary base, PKD-2 is loaded onto the ciliary membrane followed by distribution by an IFT-independent manner. Phosphorylated and/or ubiquitinated PKD-2 proteins are readily removed from the cilium via endosomal STAM-1/Hrs complexes for lysosomal degradation. IFT-dependent signals and a Kinesin-3 KLP-6 may regulate PKD-2 levels in cilia, although the site of action is yet to be determined.
Similar articles
- A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans.
Kaplan JM, Horvitz HR. Kaplan JM, et al. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2227-31. doi: 10.1073/pnas.90.6.2227. Proc Natl Acad Sci U S A. 1993. PMID: 8460126 Free PMC article. - The conserved proteins CHE-12 and DYF-11 are required for sensory cilium function in Caenorhabditis elegans.
Bacaj T, Lu Y, Shaham S. Bacaj T, et al. Genetics. 2008 Feb;178(2):989-1002. doi: 10.1534/genetics.107.082453. Epub 2008 Feb 1. Genetics. 2008. PMID: 18245347 Free PMC article. - Mating behavior, male sensory cilia, and polycystins in Caenorhabditis elegans.
O'Hagan R, Wang J, Barr MM. O'Hagan R, et al. Semin Cell Dev Biol. 2014 Sep;33:25-33. doi: 10.1016/j.semcdb.2014.06.001. Epub 2014 Jun 27. Semin Cell Dev Biol. 2014. PMID: 24977333 Free PMC article. Review. - Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans.
Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Evans JE, et al. J Cell Biol. 2006 Feb 27;172(5):663-9. doi: 10.1083/jcb.200509115. Epub 2006 Feb 21. J Cell Biol. 2006. PMID: 16492809 Free PMC article. - The sensory cilia of Caenorhabditis elegans.
Inglis PN, Ou G, Leroux MR, Scholey JM. Inglis PN, et al. WormBook. 2007 Mar 8:1-22. doi: 10.1895/wormbook.1.126.2. WormBook. 2007. PMID: 18050505 Free PMC article. Review.
Cited by
- Gene Cascade Finder: A tool for identification of gene cascades and its application in Caenorhabditis elegans.
Nomoto Y, Kubota Y, Ohnishi Y, Kasahara K, Tomita A, Oshime T, Yamashita H, Fahmi M, Ito M. Nomoto Y, et al. PLoS One. 2019 Sep 10;14(9):e0215187. doi: 10.1371/journal.pone.0215187. eCollection 2019. PLoS One. 2019. PMID: 31504044 Free PMC article. - A cilia-independent function of BBSome mediated by DLK-MAPK signaling in C. elegans photosensation.
Zhang X, Liu J, Pan T, Ward A, Liu J, Xu XZS. Zhang X, et al. Dev Cell. 2022 Jun 20;57(12):1545-1557.e4. doi: 10.1016/j.devcel.2022.05.005. Epub 2022 May 31. Dev Cell. 2022. PMID: 35649417 Free PMC article. - Cutting off ciliary protein import: intraflagellar transport after dendritic femtosecond-laser ablation.
Mijalkovic J, Girard J, van Krugten J, van Loo J, Zhang Z, Loseva E, Oswald F, Peterman EJG. Mijalkovic J, et al. Mol Biol Cell. 2020 Mar 1;31(5):324-334. doi: 10.1091/mbc.E18-06-0399. Epub 2020 Jan 15. Mol Biol Cell. 2020. PMID: 31940255 Free PMC article. - Sexual Dimorphism and Sex Differences in Caenorhabditis elegans Neuronal Development and Behavior.
Barr MM, García LR, Portman DS. Barr MM, et al. Genetics. 2018 Mar;208(3):909-935. doi: 10.1534/genetics.117.300294. Genetics. 2018. PMID: 29487147 Free PMC article. - Cilia proteins getting to work - how do they commute from the cytoplasm to the base of cilia?
Hibbard JVK, Vázquez N, Wallingford JB. Hibbard JVK, et al. J Cell Sci. 2022 Sep 1;135(17):jcs259444. doi: 10.1242/jcs.259444. Epub 2022 Sep 8. J Cell Sci. 2022. PMID: 36073764 Free PMC article. Review.
References
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–37. - PubMed
- Ware RW, Clark DV, Crossland K, Russell RL. The nerve ring of the nematode Caenorhabditis elegans: Sensory input and motor output. J. Comp. Neurol. 1975;162:71–110.
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans: the mind of a worm. Phil. Trans. R. Soc. Lond. 1986;314:1–340. - PubMed
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources