Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer's disease - PubMed (original) (raw)
Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer's disease
Karine L Richard et al. J Neurosci. 2008.
Abstract
Microglia are the immune cells of the brain, they are activated in the brain of Alzheimer's disease (AD) patients and mouse models of AD, and they express the innate immune receptor toll-like receptor 2 (TLR2). The present study investigated role of this receptor in the progression of AD-like pathologies. Here we show that amyloid beta (A beta) stimulates TLR2 expression in a small proportion of microglia. We then generated triple transgenic mice that are deficient in TLR2 from mice that harbor a mutant human presenelin 1 and a chimeric mouse/human amyloid precursor protein (APP) genes. TLR2 deficiency accelerated spatial and contextual memory impairments, which correlated with increased levels of A beta(1-42) and transforming growth factor beta1 in the brain. NMDA receptors 1 and 2A expression levels were also lower in the hippocampus of APP-TLR2(-/-) mice. Gene therapy in cells of the bone marrow using lentivirus constructs expressing TLR2 rescued the cognitive impairment of APP-TLR2(-/-) mice. Indeed, lenti-green fluorescent protein/TLR2 treatment had beneficial effects by restoring the memory consolidation process disrupted by TLR2 deficiency in APP mice. These data suggest that TLR2 acts as an endogenous receptor for the clearance of toxic A beta by bone-marrow-derived immune cells. The cognitive decline is markedly accelerated in a context of TLR2 deficiency. Upregulating this innate immune receptor may then be considered as a potential new powerful therapeutic approach for AD.
Figures
Figure 1.
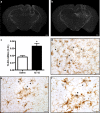
TLR2 gene expression in response to exogenous and endogenous Aβ. A solution containing either sterile saline (a) or synthetic Aβ1–42 peptide (b) was injected into the hippocampus of CD1 mice. TLR2 mRNA expression was determined by in situ hybridization (agglomeration of silver grains) 6 h after the intracerebral infusion. c, TLR2 mRNA expression levels (as determined by optical density quantification) were significantly increased in areas distal to the injection site of Aβ1–42-treated animals. *p < 0.05 (Student's t test; saline, n = 3; Aβ1–42, n = 5). d, Tissue sections were stained with an antibody directed against iba1 to reveal microglia (brown cells) and hybridized with TLR2 mRNA probe. TLR2 mRNA signal always colocalized with iba1-immunoreactive cells surrounding the injection site (indicated with arrows). e, f, Activated microglia had also a positive signal for TLR2 transcript in the brain APP mice (iba1+/TLR2+). Scale bars: a, b, 500 μm; d–f, 20 μm. Error bars indicate SEM.
Figure 2.
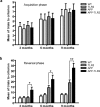
TLR2 deficiency accelerates the onset and severity of spatial memory impairment in APP/PS1 mice. a, Animals were trained to reach the hidden platform on the T-water maze apparatus, and mean of trials to accomplish the task was presented. All the different mouse groups had similar acquisition phases at 3, 6, and 9 months of age. b, The reversal learning phase was conducted 48 h later. APP–TLR2 transgenic mice exhibited a greater decline of their spatial memory capacity than their control and APP mice. *p < 0.05, **p < 0.01 (two-way ANOVA was performed revealing a significant interaction between factors age and genotype, and multiple comparisons of genotype simple effects in each level of age was performed by Bonferroni's post hoc test). Error bars indicate SEM.
Figure 3.
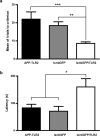
Behavioral analysis of APP–TLR2 transgenic mice unravels a contextual memory deficit. a, All mouse groups showed similar step-through latency on the single training trial of the passive avoidance test. b, Twenty-four hours after the conditioning test, APP–TLR2 mice showed significantly lower latency to enter the dark compartment of the passive avoidance apparatus. Mean latencies are depicted for each mouse groups at 3, 6, and 9 months of age. Pairwise group comparison revealed a significant main effect between APP–TLR2 and other groups of mice. ***p < 0.001; n = 9–12 (two-way ANOVA, Tamhane post hoc test). No specific pairwise comparisons could be done at each age because of lack of a group × age interaction. Error bars indicate SEM.
Figure 4.
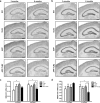
Amyloid plaque formation is delayed in the brain of APP mice that bear a mutation in the gene encoding TLR2. a–i, Anti-Aβ immunoreactivity in the brains of APP (a, d, g) and APP–TLR2 (b, e, h) mice. APP mice exhibited higher amyloid loads than APP–TLR2 mice at 3 (a, b) and 6 (d, e) months of age. g, h, Such a difference in the plaque formation was attenuated at later time points, because the surface occupied by the positive Aβ signal was similar in both groups of APP mice at 9 months of age. c, f, i, Percentage of area covered by senile plaques in the brain of mice killed at 3, 6, and 9 months, respectively. *p < 0.05, **p < 0.01 (Student's t test; APP, n = 6–11; APP–TLR2, n = 8–11). Error bars indicate SEM. Scale bars, 250 μm.
Figure 5.
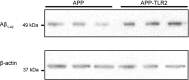
Enhanced production of Aβ1–42 in the brains of APP–TLR2 mice. Proteins from hemi-forebrains of 6-month-old APP and APP–TLR2 mice were immunoblotted with an anti-Aβ1–42 antibody. β-Actin levels were used for equal protein loading of samples.
Figure 6.
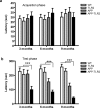
Differential expression levels for the genes encoding NMDA receptors in the hippocampus of APP and APP/TLR2 mice. Relative NMDA-R1 (a) and NMDA-R2A (b) mRNA levels were quantified in the dentate gyrus and the pyramidal cell layer of the hippocampus of WT (n = 8), TLR2 knock-out (n = 6–7), APP (n = 6–11), and APP–TLR2 (n = 9–10) mice as described in Materials and Methods. c, d, Optical densities (O.D.) normalized to the background values. Please note the significantly (*p < 0.05) lower mRNA levels for both receptors in the hippocampus of APP/TLR2 mice compared with the other mouse groups at 6 months of age. This is suggestive of potential synaptic defects of learning and memory, which was evaluated by the different behavioral tests. NMDA-R1 expression levels were also higher in the hippocampus of 3-month-old APP mice when compared with those of WT control mice (*p < 0.05). Two-way ANOVA was performed and revealed a significant interaction between factors age and genotype. Bonferroni's post hoc test was then used for multiple comparisons to determine significance levels between mouse genotypes at either 3 or 6 months of age. Error bars indicate SEM. Scale bars, 500 μm.
Figure 7.
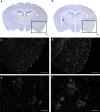
TGFβ1 mRNA expression is increased in regions surrounding Aβ plaques in the brain of TLR2-deficient APP mice. Representative dark-field photomicrograph of in situ hybridization signals showing the expression of TGFβ1 mRNA in brains of 9-month-old APP (c, e) and APP–TLR2 (d, f) mice and the corresponding thionin staining sections (a, b). Scale bars: c, d, 500 μm; e, f, 50 μm.
Figure 8.
The lentiGFP/TLR2 treatment in the bone marrow rescues spatial and contextual memory in 6-month-old APP–TLR2 mice. The mean of trials to reach the criterion during the reversal phase in the T-water maze indicates that lentiGFP/TLR2-treated mice required significantly fewer trials to learn the task than APP–TLR2 transgenic animals that were treated with the control lentiGFP virus (a). It is of great interest to note that lentiGFP/TLR2-treated APP–TLR2 transgenic animals performed comparably with wild-type mice. **p < 0.01, *** p < 0.001 (one-way ANOVA; APP–TLR2 reproduced from Fig. 2, n = 9; lentiGFP, n = 11; lentiGFP/TLR2, n = 14). The lentiGFP/TLR2-treated animals also showed a higher step-through latency to enter the dark compartment during the passive avoidance task compared with controls (b). *p < 0.05 (one-way ANOVA; APP–TLR2 reproduced from Fig. 3, n = 9; lentiGFP, n = 5; lentiGFP/TLR2, n = 7). APP–TLR2 mice received the different lentivirus preparations at 2.5–3 months of age and were tested 3 months later. For details, see Materials and Methods. Error bars indicate SEM.
Similar articles
- CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease.
Naert G, Rivest S. Naert G, et al. J Neurosci. 2011 Apr 20;31(16):6208-20. doi: 10.1523/JNEUROSCI.0299-11.2011. J Neurosci. 2011. PMID: 21508244 Free PMC article. - Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer's disease.
Aso E, Lomoio S, López-González I, Joda L, Carmona M, Fernández-Yagüe N, Moreno J, Juvés S, Pujol A, Pamplona R, Portero-Otin M, Martín V, Díaz M, Ferrer I. Aso E, et al. Brain Pathol. 2012 Sep;22(5):636-53. doi: 10.1111/j.1750-3639.2011.00560.x. Epub 2012 Jan 13. Brain Pathol. 2012. PMID: 22188425 Free PMC article. - Intranasal amyloid model of Alzheimer's disease - potential opportunities and challenges.
Singh RK. Singh RK. Pharmacol Rep. 2025 Apr;77(2):425-433. doi: 10.1007/s43440-024-00692-4. Epub 2025 Jan 8. Pharmacol Rep. 2025. PMID: 39775701 Review. - Shared cognitive and behavioral impairments in epilepsy and Alzheimer's disease and potential underlying mechanisms.
Chin J, Scharfman HE. Chin J, et al. Epilepsy Behav. 2013 Mar;26(3):343-51. doi: 10.1016/j.yebeh.2012.11.040. Epub 2013 Jan 13. Epilepsy Behav. 2013. PMID: 23321057 Free PMC article. Review.
Cited by
- Mechanisms of Aβ Clearance and Degradation by Glial Cells.
Ries M, Sastre M. Ries M, et al. Front Aging Neurosci. 2016 Jul 5;8:160. doi: 10.3389/fnagi.2016.00160. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27458370 Free PMC article. Review. - Energy metabolism and inflammation in brain aging and Alzheimer's disease.
Yin F, Sancheti H, Patil I, Cadenas E. Yin F, et al. Free Radic Biol Med. 2016 Nov;100:108-122. doi: 10.1016/j.freeradbiomed.2016.04.200. Epub 2016 May 3. Free Radic Biol Med. 2016. PMID: 27154981 Free PMC article. Review. - Therapeutic effects of glatiramer acetate and grafted CD115⁺ monocytes in a mouse model of Alzheimer's disease.
Koronyo Y, Salumbides BC, Sheyn J, Pelissier L, Li S, Ljubimov V, Moyseyev M, Daley D, Fuchs DT, Pham M, Black KL, Rentsendorj A, Koronyo-Hamaoui M. Koronyo Y, et al. Brain. 2015 Aug;138(Pt 8):2399-422. doi: 10.1093/brain/awv150. Epub 2015 Jun 6. Brain. 2015. PMID: 26049087 Free PMC article. - The Hidden Role of Non-Canonical Amyloid β Isoforms in Alzheimer's Disease.
Busch L, Eggert S, Endres K, Bufe B. Busch L, et al. Cells. 2022 Oct 29;11(21):3421. doi: 10.3390/cells11213421. Cells. 2022. PMID: 36359817 Free PMC article. Review. - Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition.
Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Chakrabarty P, et al. FASEB J. 2010 Feb;24(2):548-59. doi: 10.1096/fj.09-141754. Epub 2009 Oct 13. FASEB J. 2010. PMID: 19825975 Free PMC article.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Bi H, Sze CI. N-methyl-d-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer's disease. J Neurol Sci. 2002;200:11–18. - PubMed
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases