Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway - PubMed (original) (raw)
Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway
Yong Chen et al. J Neurosci. 2008.
Abstract
Tolerance to the analgesic effects of opioids occurs after their chronic administration, a pharmacological phenomenon that has been associated with the development of abnormal pain sensitivity such as hyperalgesia. In the present study, we investigated the role of TRPV1, which is crucial for the transduction of noxious chemical and thermal stimuli, in morphine tolerance and tolerance-associated thermal hyperalgesia. After chronic morphine treatment, a marked increase in TRPV1 immunoreactivity (IR) was detected in L4 dorsal root ganglion (DRG) neurons, spinal cord dorsal horn, and sciatic nerve. Real-time reverse transcription (RT)-PCR demonstrated that TRPV1 mRNA was upregulated in spinal cord and sciatic nerve but not in the DRG. Intrathecal pretreatment with SB366791 [N-(3-methoxyphenyl)-4-chlorocinnamide], a selective antagonist of TRPV1, attenuated both morphine tolerance and associated thermal hyperalgesia. Chronic morphine exposure induced increases in phosphorylation of mitogen-activated protein kinases (MAPKs), including p38 MAPK-IR, extracellular signal-regulated protein kinase (ERK)-IR, and c-Jun N-terminal kinase (JNK)-IR, in L4 DRG neurons. Intrathecal administration of the selective p38, ERK, or JNK inhibitors not only reduced morphine tolerance and associated thermal hyperalgesia but also suppressed the morphine-induced increase of TRPV1-IR in DRG neurons, spinal cord, and sciatic nerve and of mRNA levels in spinal cord and sciatic nerve. Together, we have identified a novel mechanism by which sustained morphine treatment results in tolerance and tolerance-associated thermal hyperalgesia, by regulating TRPV1 expression, in a MAPK-dependent manner. Thus, blocking TRPV1 might be a way to reduce morphine tolerance.
Figures
Figure 1.
Photomicrographs of TRPV1-IR in DRG (A, B), spinal cord (C, D), and sciatic nerve (E, F) from control (A, C, E) and morphine (B, D, F) treated rats. A, Arrow indicates a TRPV1-IR DRG neuron. Scale bars: (in D) A–D, 20 μm; (in F) E, F, 40 μm. Veh, 25% DMSO; Mor, Morphine.
Figure 2.
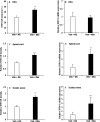
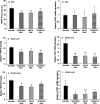
Chronic morphine increases TRPV1-IR in DRG, spinal cord, and sciatic nerve and mRNA levels in spinal cord and sciatic nerve. A, C, E, Immunohistochemistry showed an increase of TRPV1-IR in DRG neurons (A) and an enhanced density of TRPV1-IR in the spinal superficial dorsal horn (C) and in sciatic nerve (E) of chronic morphine-treated rats (***p < 0.001, Veh + Mor vs Veh + NS). B, D, F, RT-PCR revealed no significant change in TRPV1 mRNA levels in the DRGs (B) but increased TRPV1 mRNA levels in both spinal cord and sciatic nerve (D, F; ***p < 0.001, Veh + Mor vs Veh + NS) of morphine-tolerant rats. Student's t test was used for statistical analysis. n = 6 for each group.
Figure 3.
Western blot analysis of TRPV1 in DRG, spinal cord, and sciatic nerve. A, Example of Western blot with TRPV1 antibody showing bands at 95 kDa in spinal cord of morphine-tolerant and control rats. B–D, Semiquantitative density measuring revealed no significant difference in spinal cord (B), DRG (C), and sciatic nerve (D) between morphine-tolerant and control rats (Veh + Mor vs Veh + NS). β-Actin served as loading control. Student's t test was used for statistical analysis. n = 6 for each group.
Figure 4.
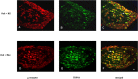
Double immunostaining of μ receptor (A, D) and TRPV1 (B, E) shows the colocalization in DRG neurons from control (A–C) and chronic morphine-treated (D–F) rats as indicated in yellow when both images are merged. Arrows in C and F indicate the DRG neurons immunoreactive for both TRPV1 and μ receptor. Scale bar (in F): A–F, 20 μm.
Figure 5.
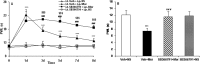
Effects of intrathecal administration of the TRPV1 inhibitor SB366791 on morphine tolerance (A) and tolerance-associated thermal hyperalgesia (B) assessed by the PWL test. Although morphine consistently produced significant antinociception until day 8 (A; ***p < 0.001, Veh + Mor vs Veh + NS), the effect gradually declined during chronic exposure from day 3 to day 8, which was accompanied by thermal hyperalgesia on day 8 (B**; ***p < 0.001, Veh + Mor vs Veh + NS). Rats in the morphine group pretreated with SB366791 displayed significantly longer PWLs from day 3 to day 8 (**_A_**; $p < 0.001 for SB366791 + Mor vs Veh + Mor, and ###p < 0.001 for SB366791 + Mor vs Veh + NS) and also had attenuated morphine-induced thermal hyperalgesia on day 8 (B; ###p < 0.001, SB366791 + Mor vs Veh + Mor). Neither Veh + NS nor SB366791 + NS treatment altered pain thresholds throughout the observation period. Two-way (A) and one-way (B**) ANOVA, followed by Tukey's test were used for statistical analysis. n = 6 for each group.
Figure 6.
Photomicrographs (A–D, F–I, K–N) and quantification (E, J, O) showing p-p38-IR (A–E), p-ERK-IR (F–J), and p-JNK-IR (K–O) in DRG neurons of the control, morphine, and the selective MAPK inhibitor pretreated control and morphine groups. Percentages of p-p38-, p-ERK-, and p-JNK-IR were increased after chronic morphine exposure (B, G, L and E, J, O; ***p < 0.001, Veh + Mor vs Veh + NS); this increase was reduced by treatment with the selective MAPK inhibitors (C, H, M and E, J, O; ###p < 0.001 for SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + Mor). No significant difference in the phosphorylation of MAPK was detected between control and the selective MAPK inhibitor pretreated control groups (SB203580 + NS, U0126 + NS, or SP600125 + NS vs Veh + NS). One-way ANOVA, followed by Tukey's test was used for statistical analysis. n = 6 for each group. Arrows in B, G, and L indicate p-p38-, p-ERK-, or p-JNK-IR DRG neurons. Scale bar (in N): A–D, F–I, K–N, 40 μm.
Figure 7.
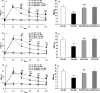
Effects of repeated administration of the selective MAPK inhibitors on morphine tolerance (A, C, E) and associated thermal hyperalgesia (B, D, F) assessed by the PWL test. Although morphine consistently produced significant antinociception until day 8 (A, C, E; ***p < 0.001, Veh + Mor vs Veh + NS), the effect gradually declined during chronic exposure from day 3 to day 8, which was accompanied by thermal hyperalgesia on day 8 (B**, D, F; ***p < 0.001, Veh + Mor vs Veh + NS). Pretreatment with the selective MAPK inhibitors, SB203580, U0126, or SP600125, produced significantly longer PWLs from day 3 to day 8 (**_A_**, **_C_**, **_E_**; $p < 0.001 for SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + Mor; ###p < 0.001 for SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + NS) and attenuated chronic morphine-induced thermal hyperalgesia on day 8 (B, D, F; ###p < 0.001, SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + Mor). None of the inhibitors altered pain thresholds in rats receiving NS throughout the observation period. Two-way (A, C, E) and one-way (B**, D, F) ANOVA, followed by Tukey's test were used for statistical analysis. n = 6 for each group.
Figure 8.
Photomicrographs of TRPV1-IR in DRG (A–D), spinal cord (E–H), and sciatic nerve (I–L) from rats treated with morphine (A, E, I) and additional pretreatment with SB203580 (B, F, J), U0126 (C, G, K), or SP600125 (D, H, L). Arrow in A indicates a TRPV1-IR DRG neuron. Scale bars: (in H) A–H, 20 μm; (in L) I–L, 40 μm.
Figure 9.
Chronic morphine increases TRPV1-IR in DRG, spinal cord, and sciatic nerve and mRNA levels in spinal cord and sciatic nerve via the MAPK signaling pathway. Chronic morphine-induced increase of TRPV1-IR in DRG neurons (A) and enhanced density of TRPV-IR in the spinal superficial dorsal horn (C) and sciatic nerve (E) was reduced by pretreatment with the selective MAPK inhibitors (*p < 0.05, **p < 0.01, and ***p < 0.001 for SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + Mor). Pretreatment with the selective MAPK inhibitors did not produce significant effects on TRPV1 mRNA levels in the DRGs (B) but greatly reduced the increase of TRPV1 mRNA levels in both spinal cord and sciatic nerve (D, F; *p < 0.05 and ***p < 0.001 for SB203580 + Mor, U0126 + Mor, or SP600125 + Mor vs Veh + Mor) of morphine-tolerant rats. One-way ANOVA, followed by Tukey's test was used for statistical analysis. n = 6 for each group.
Similar articles
- Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation.
Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Obata K, et al. J Neurosci. 2004 Nov 10;24(45):10211-22. doi: 10.1523/JNEUROSCI.3388-04.2004. J Neurosci. 2004. PMID: 15537893 Free PMC article. - Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study.
Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Ma W, et al. Eur J Neurosci. 2001 Oct;14(7):1091-104. doi: 10.1046/j.0953-816x.2001.01731.x. Eur J Neurosci. 2001. PMID: 11683901 - A role for protein kinase C-dependent upregulation of adrenomedullin in the development of morphine tolerance in male rats.
Hong Y, Wang D, Chabot JG, Ma W, Chen P, Quirion R. Hong Y, et al. J Neurosci. 2010 Sep 15;30(37):12508-16. doi: 10.1523/JNEUROSCI.0306-10.2010. J Neurosci. 2010. PMID: 20844145 Free PMC article. - The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.
Bao Y, Gao Y, Yang L, Kong X, Yu J, Hou W, Hua B. Bao Y, et al. Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review. - The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence.
Chen Y, Sommer C. Chen Y, et al. Mol Neurobiol. 2009 Oct;40(2):101-7. doi: 10.1007/s12035-009-8074-z. Epub 2009 May 26. Mol Neurobiol. 2009. PMID: 19468867 Review.
Cited by
- Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons.
Ikeda-Miyagawa Y, Kobayashi K, Yamanaka H, Okubo M, Wang S, Dai Y, Yagi H, Hirose M, Noguchi K. Ikeda-Miyagawa Y, et al. Mol Pain. 2015 Mar 8;11:8. doi: 10.1186/s12990-015-0004-7. Mol Pain. 2015. PMID: 25889103 Free PMC article. - Antinociceptive and genotoxic assessments of the antagonist TRPV1 receptor SB-366791 on morphine-induced tolerance in mice.
Mazeto TK, Picada JN, Correa ÁP, Rebelo IN, Ribeiro MT, Gomez MV, de Souza AH. Mazeto TK, et al. Naunyn Schmiedebergs Arch Pharmacol. 2020 Mar;393(3):481-490. doi: 10.1007/s00210-019-01748-6. Epub 2019 Oct 26. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31655852 - CNGA3: a target of spinal nitric oxide/cGMP signaling and modulator of inflammatory pain hypersensitivity.
Heine S, Michalakis S, Kallenborn-Gerhardt W, Lu R, Lim HY, Weiland J, Del Turco D, Deller T, Tegeder I, Biel M, Geisslinger G, Schmidtko A. Heine S, et al. J Neurosci. 2011 Aug 3;31(31):11184-92. doi: 10.1523/JNEUROSCI.6159-10.2011. J Neurosci. 2011. PMID: 21813679 Free PMC article. - TRPV1 Channel: A Potential Drug Target for Treating Epilepsy.
Nazıroğlu M. Nazıroğlu M. Curr Neuropharmacol. 2015;13(2):239-47. doi: 10.2174/1570159x13666150216222543. Curr Neuropharmacol. 2015. PMID: 26411767 Free PMC article. Review. - Downregulation of miR-219 enhances brain-derived neurotrophic factor production in mouse dorsal root ganglia to mediate morphine analgesic tolerance by upregulating CaMKIIγ.
Hu XM, Cao SB, Zhang HL, Lyu DM, Chen LP, Xu H, Pan ZQ, Shen W. Hu XM, et al. Mol Pain. 2016 Sep 5;12:1744806916666283. doi: 10.1177/1744806916666283. Print 2016. Mol Pain. 2016. PMID: 27599867 Free PMC article.
References
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. - PubMed
- Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–132. - PubMed
- Burnette WM. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein. Anal Biochem. 1981;112:195–203. - PubMed
- Cao JL, He JH, Ding HL, Zeng YM. Activation of the spinal ERK signaling pathway contributes naloxone-precipitated withdrawal in morphine-dependent rats. Pain. 2005;118:336–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous