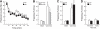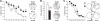Spatial relational memory requires hippocampal adult neurogenesis - PubMed (original) (raw)
Spatial relational memory requires hippocampal adult neurogenesis
David Dupret et al. PLoS One. 2008.
Abstract
The dentate gyrus of the hippocampus is one of the few regions of the mammalian brain where new neurons are generated throughout adulthood. This adult neurogenesis has been proposed as a novel mechanism that mediates spatial memory. However, data showing a causal relationship between neurogenesis and spatial memory are controversial. Here, we developed an inducible transgenic strategy allowing specific ablation of adult-born hippocampal neurons. This resulted in an impairment of spatial relational memory, which supports a capacity for flexible, inferential memory expression. In contrast, less complex forms of spatial knowledge were unaltered. These findings demonstrate that adult-born neurons are necessary for complex forms of hippocampus-mediated learning.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
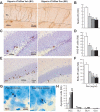
Figure 1. Inducible expression of Bax proteins in hippocampal neural precursors using a Tet-On system.
(A) Schematic representation of the inducible Tet-ON system. rtTA expression is driven either in vitro by the CMV promoter in stably transfected CHO-K1 Tet-ON cells or in vivo by the rat nestin intron II enhancer/promoter. Doxycycline (Dox) activates the rtTA protein, which binds to seven TetO sequences (TRE) to drive the co-transcription of ECFPBax and EYFPBax transgenes. (B) Strategy to obtain Dox-dependent ablation of neural precursors in vivo using double transgenic nestin-rtTA/Tet-Bax mice. (C) Six hours of Dox treatment induces ECFPBax and EYFPBax expression and multimerization in CHO-K1 Tet-ON cells. The presence of ECFPBax/EYFPBax multimers at the mitochondrial membrane was confirmed by FRET analysis. (D) Number of Bax multimers in the subventricular zone (SVZ) and the subgranular zone (SGZ) of bigenic mice treated with (bigenic-Dox mice, BD) or without (bigenic-vehicle mice, BV) Dox (2 mg/ml) during a short term (3 weeks) or a long term (17 weeks) period. (E) Confocal illustration showing EYFPBax (green) clusters (arrows) in the cytoplasm of cells located in the SGZ of bigenic-Dox mice (blue = hoescht nuclear counterstaining). (F) FRET analysis of Bax multimers shows the intermolecular interaction between ECFPBax and EYFPBax fusion proteins. FRET efficiency measured following EYFPBax acceptor photobleaching results in a brightening of the ECFPBax donor fluorescence in areas devoid of (Ctrl) or containing (Bax) Bax multimers. (G) Confocal illustration showing that nestin-IR neuronal precursors (red) are positive for EYFPBax (green). Hoescht nuclear counterstaining is shown in blue. (H) Confocal illustration showing that NeuN-IR mature granule neurons (red) are negative for EYFPBax (green). (I) Number of Bax multimers in the SGZ of bigenic mice treated acutely with Dox (2 mg/ml/100 g body weight, i.p.) and sacrificed at different time intervals. ***: p≤0.001 compared to BV, +++: p≤0.001 compared to Ctrl areas, #: p≤0.05, ##: p≤0.01, ###: p≤0.001 compared to 0 h, °: p≤0.05, °°°: p≤0.001 compared to 2 h, §§§: p≤0.001 compared to 24 h.
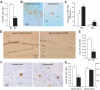
Figure 2. Effects of short-term Dox treatment on adult hippocampal neurogenesis.
(A) Illustration of nestin staining. (B) Densitometric analysis of nestin-IR. (C) Illustration of dividing cells visualized by HH3 staining. (D) Number of HH3-IR cells. (E) Illustrations of newborn cells visualized by BrdU staining. (F) Number of BrdU-IR cells. (G) Illustrations of apoptotic cells. (H) Number of apoptotic cells in the subgranular zone where newborn cells are produced (SGZ) and in the internal (GLi), medial (GLm) and external (GLe) granular cell layer. GL = granular cell layer. *: p≤0.05, **: p≤0.01, ***: p≤0.001 in comparison to the bigenic-vehicle group. ##: p≤0.01, in comparison to bigenic fed with Dox at 1 mg/ml.
Figure 3. Effects of long-term Dox treatment on adult neurogenesis.
(A) Number of HH3-IR cells in the dentate gyrus (DG) of bigenic mice treated with (BD, black) or without (BV, white) Dox. (B) Illustration of Caspase 3-IR cells in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the DG. (C) Density of Caspase 3-IR cells in the SVZ and the SGZ of bigenic (black) and control mice (white) treated with Dox. (D) Illustrations of immature neurons visualized by Dcx staining in the DG. (E) Number of Dcx-IR immature neurons in the DG of bigenic mice treated with (black) or without (white) Dox. (F) Number of one-month-old BrdU-IR cells in the DG and the OB of bigenic (black) and control mice (white) treated with Dox. **: p≤0.01 and ***: p≤0.001 compared to control groups.
Figure 4. Diagram of behavioral task sequences.
Figure 5. Effects of adult hippocampal neurogenesis ablation on simple forms of spatial knowledge.
(A) Exploration in a novel environment measured by photocell beam breaks. (B) Contextual fear conditioning assessed by the percentage of freezing displayed by C57BL/6J wild-type (Wt) mice before conditioning (Base) and when re-exposed 24 hours later to the conditioning context (Cond.Ctx). Mice were infused with Vehicle (Veh) or lidocaine (Lido) in the dorsal hippocampus before conditioning. (C) Contextual fear conditioning assessed by the percentage of freezing displayed by control (CD) and bigenic mice (BD) treated with doxycycline in the basal condition and when re-exposed to the conditioning context 24 hours later. (D) Fear conditioning assessed by the percentage of freezing displayed 24 hours after conditioning by CD and BD mice when exposed to a neutral context (Neut.Ctx) before (Pre-tone) and during (Tone) re-exposure to the tone present during conditioning but unpaired with the shock. BV = bigenic-vehicle mice. °°°: p≤0.001 compared to Base. *: p≤0.05 compared to Wt-Veh.
Figure 6. Effects of adult hippocampal neurogenesis ablation on spatial navigation.
(A) Latency to reach the hidden platform using variable start positions. Right: representative swim paths during the last training day (V9) for a bigenic mouse treated with Dox (BD) and a bigenic mouse treated with vehicle (BV). (B) Time spent in the target quadrant during the probe test. Right: representative swim paths during the probe test. (C) Latency to reach the hidden platform using constant (C1 to C8) or novel (N) start positions. Right: representative swim paths during constant (C8) and novel (N) start position training days. BV = bigenic-vehicle mice; BD = bigenic-Dox mice; CD = control-Dox mice. ###: p≤0.001 compared to C8; **: p≤0.01, ***: p≤0.001 compared to the control group; +++: p≤0.001 compared to chance level. Black arrows indicate start point positions.
Similar articles
- Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation.
Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Sahay A, et al. Nature. 2011 Apr 28;472(7344):466-70. doi: 10.1038/nature09817. Epub 2011 Apr 3. Nature. 2011. PMID: 21460835 Free PMC article. - Trim9 Deletion Alters the Morphogenesis of Developing and Adult-Born Hippocampal Neurons and Impairs Spatial Learning and Memory.
Winkle CC, Olsen RH, Kim H, Moy SS, Song J, Gupton SL. Winkle CC, et al. J Neurosci. 2016 May 4;36(18):4940-58. doi: 10.1523/JNEUROSCI.3876-15.2016. J Neurosci. 2016. PMID: 27147649 Free PMC article. - A functional role for adult hippocampal neurogenesis in spatial pattern separation.
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. Clelland CD, et al. Science. 2009 Jul 10;325(5937):210-3. doi: 10.1126/science.1173215. Science. 2009. PMID: 19590004 Free PMC article. - [A new chapter in the field of memory: hippocampal neo-neurogenesis].
Dupret D, Abrous DN. Dupret D, et al. Biol Aujourdhui. 2010;204(2):113-29. doi: 10.1051/jbio/2010004. Epub 2010 Jun 21. Biol Aujourdhui. 2010. PMID: 20950556 Review. French. - Review: adult neurogenesis contributes to hippocampal plasticity.
Toda T, Gage FH. Toda T, et al. Cell Tissue Res. 2018 Sep;373(3):693-709. doi: 10.1007/s00441-017-2735-4. Epub 2017 Nov 29. Cell Tissue Res. 2018. PMID: 29185071 Review.
Cited by
- Chronic Supplementation with a Mix of Salvia officinalis and Salvia lavandulaefolia Improves Morris Water Maze Learning in Normal Adult C57Bl/6J Mice.
Dinel AL, Lucas C, Guillemet D, Layé S, Pallet V, Joffre C. Dinel AL, et al. Nutrients. 2020 Jun 15;12(6):1777. doi: 10.3390/nu12061777. Nutrients. 2020. PMID: 32549250 Free PMC article. - Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function.
Fang F, Xue Z, Cang J. Fang F, et al. Neurosci Bull. 2012 Oct;28(5):499-508. doi: 10.1007/s12264-012-1260-4. Epub 2012 Sep 11. Neurosci Bull. 2012. PMID: 22965743 Free PMC article. - Ageing, Cognitive Decline, and Effects of Physical Exercise: Complexities, and Considerations from Animal Models.
Caruso MG, Nicolas S, Lucassen PJ, Mul JD, O'Leary OF, Nolan YM. Caruso MG, et al. Brain Plast. 2024 May 14;9(1-2):43-73. doi: 10.3233/BPL-230157. eCollection 2024. Brain Plast. 2024. PMID: 38993577 Free PMC article. Review. - Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase.
Pan YW, Storm DR, Xia Z. Pan YW, et al. Neurobiol Learn Mem. 2013 Oct;105:81-92. doi: 10.1016/j.nlm.2013.07.011. Epub 2013 Jul 18. Neurobiol Learn Mem. 2013. PMID: 23871742 Free PMC article. - Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer's disease.
Kazim SF, Iqbal K. Kazim SF, et al. Mol Neurodegener. 2016 Jul 11;11(1):50. doi: 10.1186/s13024-016-0119-y. Mol Neurodegener. 2016. PMID: 27400746 Free PMC article. Review.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. - PubMed
- Scharfman HE. The Dentate Gyrus: a comprehensive guide to structure, function, and clinical implication. Elsevier. 2007:823.
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical