Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice - PubMed (original) (raw)
Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice
Fumiaki Kojima et al. J Immunol. 2008.
Abstract
Microsomal PGE synthase-1 (mPGES-1) is an inducible enzyme that acts downstream of cyclooxygenase and specifically catalyzes the conversion of PGH(2) to PGE(2). The present study demonstrates the effect of genetic deletion of mPGES-1 on the developing immunologic responses and its impact on the clinical model of bovine collagen-induced arthritis. mPGES-1 null and heterozygous mice exhibited decreased incidence and severity of arthritis compared with wild-type mice in a gene dose-dependent manner. Histopathological examination revealed significant reduction in lining hyperplasia and tissue destruction in mPGES-1 null mice compared with their wild-type littermates. mPGES-1 deficient mice also exhibited attenuation of mechanical nociception in a gene dose-dependent manner. In addition, mPGES-1 null and heterozygous mice showed a marked reduction of serum IgG against type II collagen, including subclasses IgG1, IgG2a, IgG2b, IgG2c, and IgG3, compared with wild-type mice, which correlated with the reduction in observed inflammatory features. These results demonstrate for the first time that deficiency of mPGES-1 inhibits the development of collagen-induced arthritis, at least in part, by blocking the development of a humoral immune response against type II collagen. Pharmacologic inhibition of mPGES-1 may therefore impact both the inflammation and the autoimmunity associated with human diseases such as rheumatoid arthritis.
Figures
FIGURE 1
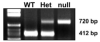
Genotyping of mPGES-1 WT, Het, and null mice by PCR analysis. The lower band (412 bp) is amplified from the WT alleles and the upper band (720 bp) is from the mPGES-1 null alleles.
FIGURE 2
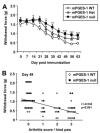
Effects of mPGES-1 gene deletion on CIA response. Time course of incidence of arthritis (A), arthritis scores (B), paw edema (C), and body weight (D) of mPGES-1 WT (n = 21), Het (n = 27), and null (n = 22) mice in CIA at the indicated days after the first immunization. Data for incidence, arthritis scores, and body weight are expressed as the mean ± SEM from each animal. Data for paw edema are expressed as the mean ± SEM from each hind paw. Single, double, and triple characters indicate significance at p < 0.05, p < 0.01, and p < 0.001, respectively. *, WT vs null; $, WT vs Het; #, Het vs null.
FIGURE 3
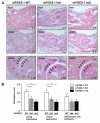
Histological analysis of arthritis severity in mPGES-1 null mice. A, Hind paws of mPGES-1 WT, Het, and null mice were collected at day 65 after the first immunization and sections were stained by H&E. Results are representative examples in mPGES-1 WT (left column), Het (middle column), and null (right column) mice with CIA. Top and middle rows show ×4 (original magnification) views of footpads and ankle joints, respectively; bottom row shows ×10 (original magnification) views of the ankle joints. Arrowheads and stars in the bottom row show enhanced cartilage destruction and hyperplasia of synovium, respectively, in WT mice compared with Het and null mice. B, Histological scores in mPGES-1 WT (open bar, n = 12), Het (gray bar, n = 17), and null (filled bar, n = 9) mice. Sections were blindly examined for lining hyperplasia, tissue destruction, and inflammatory cell infiltration. Data are expressed as the mean ± SEM. *, p < 0.05 indicates significance compared with WT mice.
FIGURE 4
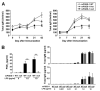
Inhibitory effect of mPGES-1 gene deletion on mechanical nociception. A, Time course of mechanical nociception in mPGES-1 WT, Het, and null mice with CIA. Values show the change in mechanical force required to elicit a withdrawal response in hind paws of mPGES-1 WT (n = 15), Het (n = 19), and null (n = 16) mice with CIA at the indicated days after the first immunization. Data are expressed as the mean ± SEM from each hind paw. Single, double, and triple characters indicate significance at p < 0.05, p < 0.01, and p < 0.001, respectively. *, WT vs null; $, WT vs Het. B, Correlation between mechanical nociception and arthritis score in mPGES-1 WT (n = 15) and null (n = 16) mice with CIA at day 49. The mechanical force required to elicit a withdrawal response is plotted against the arthritis score of individual paws at day 49. The correlation was analyzed by Spearman’s rank test and correlation coefficient (r) and p value are indicated.
FIGURE 5
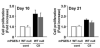
Induction of mPGES-1 and COX-2 in spleen and lymph nodes of CII/CFA-immunized mice. mRNA expression of mPGES-1 and COX-2 in WT spleen (A) and lymph nodes (B) at days 0 (control nonimmunized mice), 10, and 21 after immunization was determined by RT-PCR. Results are representative examples from 3-6 mice at each time point (top panel). Levels of mRNA expression were normalized against hypoxanthine phosphoribosyltransferase (HPRT); data are shown as the fold induction relative to day 0 expression (assigned the value “1”) and are the mean ± SEM from 3-6 mice at each time point. *, p < 0.05, indicates statistical significance compared with day 0.
FIGURE 6
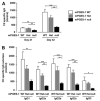
Reduction of CII-specific IgG levels in mPGES-1 deficiency. A, Levels of CII-specific total IgG in serum. Serum from mPGES-1 WT (open bar, n = 21), Het (gray bar, n = 27), and null (filled bar, n = 22) mice with CIA was collected on days 21 and 42, and titers of CII specific total IgG were measured by ELISA. B, Levels of CII-specific IgG subclasses in serum. Titers of CII-specific IgG in serum of mPGES-1 WT (open bar, n = 21), Het (gray bar, n = 27), and null (filled bar, n = 22) mice at day 42 were measured by ELISA. Data are expressed as the mean ± SEM in arbitrary units for CII-specific IgG, IgG1, IgG2a, IgG2b, IgG2c, and IgG3. *, Single, double and triple characters indicate significance at p < 0.05, p < 0.01, and p < 0.001, respectively.
FIGURE 7
CII-induced proliferation of splenocytes with mPGES-1 deficiency. Splenocytes were isolated from mPGES-1, WT, and null mice at days 10 and 21 postimmunization with CII in CFA. Cells were stimulated with CII (100 μg/ml) for 48 h and then further incubated with BrdU for 18 h. Proliferative activity was estimated from the nuclear incorporation of BrdU as measured by ELISA. Data for mPGES-1 deficient and stimulated WT cells are expressed relative to the level of incorporation in unstimulated WT splenocytes (assigned the value “1”), and are the mean ± SEM from three mPGES-1 WT, and null mice at each time point. cont, Control.
FIGURE 8
Effect of mPGES-1 gene deletion on total IgG and IgM production in vivo and in vitro. A, Serum from mPGES-1 WT (n = 6), Het (n = 8), and null (n = 6) mice with CIA were collected at the indicated days after the first immunization, and levels of IgG and IgM were measured by ELISA. Data are expressed as the mean ± SEM. Single and double characters indicate significance at p < 0.05 and p < 0.01, respectively. *, WT vs null; $, WT vs Het; #, Het vs null. B, Splenocytes isolated from mPGES-1 WT and null mice were cultured with or without LPS (0.001-10 μg/ml) for 4 days in vitro. Levels of PGE2 and total IgG and IgM in culture medium were measured by ELISA. Data are expressed as the mean ± SEM from 3-5 mPGES-1 WT and null mice. ***, p < 0.001 indicates significance.
Similar articles
- Reduced T cell-dependent humoral immune response in microsomal prostaglandin E synthase-1 null mice is mediated by nonhematopoietic cells.
Kojima F, Frolov A, Matnani R, Woodward JG, Crofford LJ. Kojima F, et al. J Immunol. 2013 Nov 15;191(10):4979-88. doi: 10.4049/jimmunol.1301942. Epub 2013 Oct 14. J Immunol. 2013. PMID: 24127557 Free PMC article. - Anti-inflammatory properties of prostaglandin E2: deletion of microsomal prostaglandin E synthase-1 exacerbates non-immune inflammatory arthritis in mice.
Frolov A, Yang L, Dong H, Hammock BD, Crofford LJ. Frolov A, et al. Prostaglandins Leukot Essent Fatty Acids. 2013 Oct;89(5):351-8. doi: 10.1016/j.plefa.2013.08.003. Epub 2013 Aug 30. Prostaglandins Leukot Essent Fatty Acids. 2013. PMID: 24055573 Free PMC article. - Shunting of prostanoid biosynthesis in microsomal prostaglandin E synthase-1 null embryo fibroblasts: regulatory effects on inducible nitric oxide synthase expression and nitrite synthesis.
Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Kapoor M, et al. FASEB J. 2006 Nov;20(13):2387-9. doi: 10.1096/fj.06-6366fje. Epub 2006 Oct 3. FASEB J. 2006. PMID: 17023389 Free PMC article. - MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis.
Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. Hegen M, et al. J Immunol. 2006 Aug 1;177(3):1913-7. doi: 10.4049/jimmunol.177.3.1913. J Immunol. 2006. PMID: 16849504 - Prostaglandin E synthase in the pathophysiology of arthritis.
Kojima F, Kato S, Kawai S. Kojima F, et al. Fundam Clin Pharmacol. 2005 Jun;19(3):255-61. doi: 10.1111/j.1472-8206.2005.00316.x. Fundam Clin Pharmacol. 2005. PMID: 15910650 Review.
Cited by
- Analgesic effects of a highly selective mPGES-1 inhibitor.
Stewart MJ, Weaver LM, Ding K, Kyomuhangi A, Loftin CD, Zheng F, Zhan CG. Stewart MJ, et al. Sci Rep. 2023 Feb 27;13(1):3326. doi: 10.1038/s41598-023-30164-3. Sci Rep. 2023. PMID: 36849491 Free PMC article. - Facilitation of colonic T cell immune responses is associated with an exacerbation of dextran sodium sulfate-induced colitis in mice lacking microsomal prostaglandin E synthase-1.
Kojima F, Sekiya H, Hioki Y, Kashiwagi H, Kubo M, Nakamura M, Maehana S, Imamichi Y, Yuhki KI, Ushikubi F, Kitasato H, Ichikawa T. Kojima F, et al. Inflamm Regen. 2022 Jan 4;42(1):1. doi: 10.1186/s41232-021-00188-1. Inflamm Regen. 2022. PMID: 34983695 Free PMC article. - Clinical data mining reveals analgesic effects of lapatinib in cancer patients.
Zhou S, Zheng F, Zhan CG. Zhou S, et al. Sci Rep. 2021 Feb 11;11(1):3528. doi: 10.1038/s41598-021-82318-w. Sci Rep. 2021. PMID: 33574423 Free PMC article. - Anti-Inflammatory Drugs as Anticancer Agents.
Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, Filosa R, Caraglia M. Zappavigna S, et al. Int J Mol Sci. 2020 Apr 9;21(7):2605. doi: 10.3390/ijms21072605. Int J Mol Sci. 2020. PMID: 32283655 Free PMC article. Review. - Crth2 receptor signaling down-regulates lipopolysaccharide-induced NF-κB activation in murine macrophages via changes in intracellular calcium.
Diwakar BT, Yoast R, Nettleford S, Qian F, Lee TJ, Berry S, Huffnagle I, Rossi RM, Trebak M, Paulson RF, Prabhu KS. Diwakar BT, et al. FASEB J. 2019 Nov;33(11):12838-12852. doi: 10.1096/fj.201802608R. Epub 2019 Sep 13. FASEB J. 2019. PMID: 31518163 Free PMC article.
References
- Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. - PubMed
- Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E2 production is regulated by the glutathione-dependent prostaglandin E2 synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J. Immunol. 2002;168:5811–5816. - PubMed
- Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor γ: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway. J. Biol. Chem. 2007;282:5356–5366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY 14060/EY/NEI NIH HHS/United States
- R01 AR049010/AR/NIAMS NIH HHS/United States
- R01 EY014060/EY/NEI NIH HHS/United States
- R01 AR049010-05/AR/NIAMS NIH HHS/United States
- R01 AR 049010/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases