MMP19 is essential for T cell development and T cell-mediated cutaneous immune responses - PubMed (original) (raw)
MMP19 is essential for T cell development and T cell-mediated cutaneous immune responses
Inken M Beck et al. PLoS One. 2008.
Abstract
Matrix metalloproteinase-19 (MMP19) affects cell proliferation, adhesion, and migration in vitro but its physiological role in vivo is poorly understood. To determine the function of MMP19, we generated mice deficient for MMP19 by disrupting the catalytic domain of mmp19 gene. Although MMP19-deficient mice do not show overt developmental and morphological abnormalities they display a distinct physiological phenotype. In a model of contact hypersensitivity (CHS) MMP19-deficient mice showed impaired T cell-mediated immune reaction that was characterized by limited influx of inflammatory cells, low proliferation of keratinocytes, and reduced number of activated CD8(+) T cells in draining lymph nodes. In the inflamed tissue, the low number of CD8(+) T cells in MMP19-deficient mice correlated with low amounts of proinflammatory cytokines, especially lymphotactin and interferon-inducible T cell alpha chemoattractant (I-TAC). Further analyses showed that T cell populations in the blood of immature, unsensitized mice were diminished and that this alteration originated from an altered maturation of thymocytes. In the thymus, thymocytes exhibited low proliferation rates and the number of CD4(+)CD8(+) double-positive cells was remarkably augmented. Based on the phenotype of MMP19-deficient mice we propose that MMP19 is an important factor in cutaneous immune responses and influences the development of T cells.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
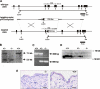
Figure 1. Generation of MMP19−/− mice.
(A) Schematic representation of the murine mmp19 gene and its exon/intron-organization as previously described by Mueller et al. ; pPNT targeting vector construct, and the resulting deleted active site locus of the mouse mmp19 gene are depicted. The targeting construct based on the pPNT vector , was generated by replacement of 1088 bp region of mmp19 gene spanning the end of exon 3 and the whole exon 4 encoding the catalytic domain by the neomycin resistance cassette. Homologous recombination led to introduction of the PGK-Neo cassette and allowed selection of homologous recombinants. The 3′-probe used for detection of replacement events is indicated by thick bars. Also shown are restriction sites used for southern hybridization screening as well as primer binding sites used for diagnostic PCR. Screening for replacement mutants employed restriction digestion of genomic DNA with _Stu_I and _Eco_RV and southern hybridization with the described 3′-probe. (B) For screening of MMP19-deficient mice Southern blot analyses were performed: mouse DNA digested with _Stu_I and _Eco_RV was probed with the diagnostic 3′-probe. Probing led to identification of either a 7.3 kb band (wild-type allele, +/+) or a 5 kb band for the targeted allele (−/−). In heterozygous mice (+/−) both alleles are present. (C) Genotyping of targeted alleles using PCR. Wild-type alleles are detected by an 800 bp band, while PCR for the MMP19-deficient allele results in a 600 bp product. (D) Primary keratinocytes isolated from wild-type, heterozygous, and homozygous MMP19-deficient mice were analyzed for MMP19 expression by western blotting using anti-MMP19 antibodies purified against a peptide derived from the hinge region of murine MMP19, that is deteced in size of 59 kD. (E) Immunohistochemical analysis of murine skin with anti-MMP19 antibodies described above. Scale bars: 50 µm.
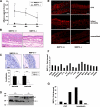
Figure 2. MMP19−/− mice show impaired ear swelling and inflammatory reaction in CHS.
(A) Five days after abdominal sensitization with the hapten (FITC), mouse ears were challenged with FITC and ear thickness was measured after 24, 48, and 72 h. Mean values are given in mm as difference to time point 0 h. (B) Hematoxylin-eosin staining of ear sections 24 h after challenge shows reduced influx of neutrophils and eosinophils in MMP19−/− mice. (C) Staining with anti-Ki-67 antibody revealed that proliferation of keratinocytes in MMP19-deficient mice was strongly reduced in basal and suprabasal layers. Ki-67-positive cells were counted and calculated as percentage of basal and total keratinocytes. Six images (magnification 400x) per mice were analyzed. (D) Decreased processing of IGFBP-3 in MMP19-deficient mice. Primary keratinocytes from MMP19+/− and MMP19−/− mice were grown for 72 h and conditioned media were analyzed for IGFBP-3 proteolysis by western blotting. The arrowhead indicates the position of intact IGFBP-3, whereas the arrow points to 30 kD IGFBP-3 proteolytic fragment. (E) Anti-CD8 staining (red) of ear sections. MMP19−/− mice show low numbers of CD8+ T cells in CHS (upper panel) as well as in a T cell-independent model of inflammation, i.e. irritant dermatitis, induced by croton oil (middle panel). Ears of unsensitized mice (lower panel) exhibit low numbers of CD8+ cells; no difference was observed between MMP19+/+ and MMP19−/− mice. CHS and irritant dermatitis were carried out in four independent experiments each with 4 wild-type and 4 MMP19−/− mice. (F) 24 h after FITC challenge ear lysates were analyzed for cytokine expression that was generally reduced in MMP19-deficient mice compared to wild-type animals. (G) Reduced levels of lymphotactin and I-TAC from three independent experiments are shown. Bars in F and G represent values of MMP19−/− mice given as fold decrease to wild-type mice. Significant values with *p<0.05 and **p<0.01; student's t-test. Scale bars: B, 50 µm; C, 20 µm; D, 50 µm.
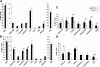
Figure 3. MMP19−/− mice exhibit reduced T cell activation in CHS.
Using flow cytometry cells of inguinal (A and B) or draining lymph nodes (C and D) from wild-type (+/+) and MMP19-deficient (−/−) mice were analyzed for the indicated activation markers, all gated on CD8+ T cells. (A) Unsensitized MMP19−/− mice show slight decrease of T cells positive for CD62L and CD95/CD25. (B) MMP19−/− mice analyzed 24 h after abdominal FITC painting (sensitization) exhibit higher numbers of naive T cells (CD62L+) and decreased numbers of activated T cells compared to MMP19+/+ mice. (C) In CHS reaction 48 h after FITC challenge on ears, draining lymph nodes of MMP19−/− mice show significantly reduced numbers of cells positive for activation and memory markers. (D) Reduced numbers of CD25+ and high numbers of CD62L+ cells are still present in MMP19−/− mice after 72 h while other activation markers were comparable to those of MMP19+/+ mice. In A and B two scales are used to match relevant data from an identical experiment. Significant values (p<0.05) are marked by asterisk. Each analysis was carried out four times with MMP19+/+ (n = 4) and MMP19−/− mice (n = 4) per experiment.
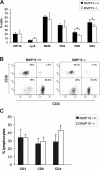
Figure 4. T cell population in blood of immature MMP19−/− mice is distorted.
(A) Blood samples of 3-weeks old MMP19+/+ and MMP19−/− mice were analyzed for monocytes, granulocytes (CD11b and Ly-6), B cells (B220), and T cell populations by flow cytometry. MMP19−/− mice exhibit significantly reduced numbers of CD4+ and CD8+ T cells. (B) A typical dot plot analysis of CD4+ and CD8+ populations gated on CD3+ cells in individual mice is shown. (C) No differences in T cell subpopulations were measured in blood of adult mice (12 weeks old). CD4+ and CD8+ T cells shown in A and C are also positive for CD3. Analyses were carried out in five independent experiments with MMP19+/+ (n = 4) and MMP19−/− mice (n = 4). Numbers of B and T lymphocytes were analyzed by gating the lymphocyte region. Significances with *p<0.05; student's t-test.
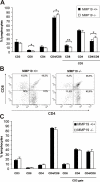
Figure 5. Development of single positive lymphocytes in the thymus of MMP19−/− mice is impeded.
Flow cytometry analysis of thymocytes from immature (3-weeks old, A and B) and adult (8–12 weeks, C) MMP19+/+ and MMP19−/− mice. (A) Young MMP19−/− mice have high numbers of CD4+/CD8+ T cells and lower numbers of CD8 single-positive thymocytes compared to MMP19+/+ mice. (B) A typical dot plot analysis of CD4+ and CD8+ T cell populations gated on CD3+ cells shows a decrease of single-positive and accumulation of double-positive T cells in thymi of MMP19−/− mice. (C) Adult MMP19+/+ and MMP19−/− mice show equal numbers of T cell subsets in the thymus. Analyses were done in four independent experiments with MMP19+/+ (n = 4) and MMP19−/− mice (n = 4). Significant values with *p<0.05 and **p<0.01; student's t-test.
Figure 6. Thymocytes of MMP19−/− mice exhibit strongly reduced proliferation.
(A) Immature MMP19−/− mice exhibit low numbers of Ki-67+ medullar cells in the thymus. Scale bars: 50 µm. (B and C). To quantify proliferating thymocytes mice were injected i.p. with BrdU. After 14 h, CD3+, CD4+, and CD8+ thymocytes were analyzed for BrdU incorporation using flow cytometry. Grey, isotype control; light red, MMP19−/− (n = 4); red, MMP19+/+ (n = 4). (C) Quantification of BrdU positive thymocytes. CD3+, CD4+, and CD8+ thymocytes from MMP19−/− mice exhibit markedly diminished proliferation. Black bars: MMP19+/+, white bars: MMP19−/−. (D) Quantitative RT-PCR analysis of MMP19 in the thymus shows expression in WT mice and confirmed the absence in MMP19−/− mice. The expression of MMP19 in the thymus is lower compared to that in the liver. CT, cycle of threshold. Significances with **p<0,01 (student's t-test).
Similar articles
- IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking.
Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. Dufour JH, et al. J Immunol. 2002 Apr 1;168(7):3195-204. doi: 10.4049/jimmunol.168.7.3195. J Immunol. 2002. PMID: 11907072 - Deficient contact hypersensitivity reaction in CD4-/- mice is because of impaired hapten-specific CD8+ T cell functions.
Saint-Mezard P, Chavagnac C, Vocanson M, Kehren J, Rozières A, Bosset S, Ionescu M, Dubois B, Kaiserlian D, Nicolas JF, Bérard F. Saint-Mezard P, et al. J Invest Dermatol. 2005 Mar;124(3):562-9. doi: 10.1111/j.0022-202X.2005.23567.x. J Invest Dermatol. 2005. PMID: 15737197 - Catalytic activity of Matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma.
Chan KC, Ko JM, Lung HL, Sedlacek R, Zhang ZF, Luo DZ, Feng ZB, Chen S, Chen H, Chan KW, Tsao SW, Chua DT, Zabarovsky ER, Stanbridge EJ, Lung ML. Chan KC, et al. Int J Cancer. 2011 Oct 15;129(8):1826-37. doi: 10.1002/ijc.25855. Epub 2011 Apr 1. Int J Cancer. 2011. PMID: 21165953 - Molecular characterization of porcine MMP19 and MMP23B genes and its association with immune traits.
Zhao S, Zhao Y, Niu P, Wang N, Tang Z, Zan L, Li K. Zhao S, et al. Int J Biol Sci. 2011;7(8):1101-13. doi: 10.7150/ijbs.7.1101. Epub 2011 Sep 14. Int J Biol Sci. 2011. PMID: 21927579 Free PMC article. - The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis.
Saint-Mezard P, Berard F, Dubois B, Kaiserlian D, Nicolas JF. Saint-Mezard P, et al. Eur J Dermatol. 2004 May-Jun;14(3):131-8. Eur J Dermatol. 2004. PMID: 15246935 Review.
Cited by
- A Tale of Two Proteolytic Machines: Matrix Metalloproteinases and the Ubiquitin-Proteasome System in Pulmonary Fibrosis.
Roque W, Boni A, Martinez-Manzano J, Romero F. Roque W, et al. Int J Mol Sci. 2020 May 29;21(11):3878. doi: 10.3390/ijms21113878. Int J Mol Sci. 2020. PMID: 32485920 Free PMC article. Review. - Transcriptomic Profiling of the Adaptive and Innate Immune Responses of Atlantic Salmon to Renibacterium salmoninarum Infection.
Eslamloo K, Caballero-Solares A, Inkpen SM, Emam M, Kumar S, Bouniot C, Avendaño-Herrera R, Jakob E, Rise ML. Eslamloo K, et al. Front Immunol. 2020 Oct 28;11:567838. doi: 10.3389/fimmu.2020.567838. eCollection 2020. Front Immunol. 2020. PMID: 33193341 Free PMC article. - Thymocyte development in the absence of matrix metalloproteinase-9/gelatinase B.
Gounko NV, Martens E, Opdenakker G, Rybakin V. Gounko NV, et al. Sci Rep. 2016 Jul 19;6:29852. doi: 10.1038/srep29852. Sci Rep. 2016. PMID: 27432536 Free PMC article. - Metalloproteinases and their natural inhibitors in inflammation and immunity.
Khokha R, Murthy A, Weiss A. Khokha R, et al. Nat Rev Immunol. 2013 Sep;13(9):649-65. doi: 10.1038/nri3499. Nat Rev Immunol. 2013. PMID: 23969736 Review. - MMPs regulate both development and immunity in the tribolium model insect.
Knorr E, Schmidtberg H, Vilcinskas A, Altincicek B. Knorr E, et al. PLoS One. 2009;4(3):e4751. doi: 10.1371/journal.pone.0004751. Epub 2009 Mar 9. PLoS One. 2009. PMID: 19270735 Free PMC article.
References
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. - PubMed
- Sedlacek R, Mauch S, Kolb B, Schatzlein C, Eibel H, et al. Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology. 1998;198:408–23. - PubMed
- Cossins J, Dudgeon TJ, Catlin G, Gearing AJ, Clements JM. Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochem Biophys Res Commun. 1996;228:494–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials