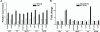The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration - PubMed (original) (raw)
The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration
Huiyi Chen et al. PLoS One. 2008.
Abstract
Background: Although the statement that age is the greatest risk factor for Age-related macular degeneration (AMD) is widely accepted, the cellular and molecular explanations for that clinical statement are not generally known. A major focus of AMD research is the retinal pigment epithelium (RPE)/choroid. The purpose of this study was to characterize the changes in the RPE/choroid with age that may provide a background for the development of AMD.
Methodology/principal findings: We compared the transcriptional profiles, key protein levels and histology of the RPE/choroid from young and old mice. Using three statistical methods, microarray data demonstrated marked changes in the old mouse. There were 315 genes differentially expressed with age; most of these genes were related to immune responses and inflammatory activity. Canonical pathways having significant numbers of upregulated genes in aged RPE/choroid included leukocyte extravasation, complement cascades, natural killer cell signaling and IL-10 signaling. By contrast, the adjacent neural retina showed completely different age-related changes. The levels of proteins that participate in leukocyte extravasation and complement pathways were consistently increased in the normal, aged RPE/choroid. Furthermore, there was increased gene expression and protein levels of leukocyte attracting signal, chemokine ligand 2 (Ccl2) in aged RPE/choroid. In old animals, there was marked extravasation and accumulation of leukocytes from the choroidal circulation onto Bruch's membrane and into the RPE.
Conclusions/significance: These phenotypic changes indicate that the RPE/choroid in the normal, old mouse has become an immunologically active tissue. There are signals from the normal, aged RPE/choroid which recruit leukocytes from the circulation and activate the complement cascade. These age-related changes that occur in the RPE/choroid with age, to the extent that they occur in the human retina, may provide the background for an error in regulation of immunological activity to cause AMD to appear in an elderly individual.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
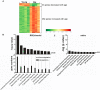
Figure 1. Changes in gene expression in RPE/choroid from old animals.
(A) The transcriptional profiles of the normal RPE/choroid from young and old mice were analyzed by hierarchical clustering of 315 differentially expressed, age-regulated genes by Limma analysis. Standardized expression values of genes are displayed according to the color scale, in which red represents above average expression and green represents below average expression. Absolute fold changes of individual genes are shown in Table S1 online. (B) Relative changes in canonical pathways in RPE/choroid from old animals. The upper figure shows the –log (p value) of the first 10 canonical pathways which changed significantly in RPE/choroid from old animals. The horizontal line represents the threshold of p which is equivalent to p = 0.05. Bars above the line indicate p<0.05. The lower part of the figure shows the number of differentially expressed genes in each pathway. (C) The –log (p value) of the 9 canonical pathways which changed significantly in neural retina from old animals.
Figure 2. Real time RT-PCR confirmation of microarray results.
(A) Relative mRNA expression levels of selected genes from the first 5 canonical pathways (see Fig. 1B) in RPE/choroid from young and old animals. (B) Comparison of the fold changes of the same selected genes determined by microarray analysis and real-time RT-PCR. LES, leukocyte extravasation signaling; CC, complement cascade; NKCS, natural killer cell signaling; ILS, IL-10 signaling; BCRS, B cell receptor signaling.
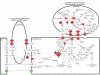
Figure 3. Pathway diagram showing the molecules involved in leukocyte extravasation signaling and their interaction.
Color nodes: genes that changed significantly in RPE/choroid from old animals with a fold change >2. Red: upregulation; green: downregulation. The diagram was modified from Ingenuity Pathway Analysis (Ingenuity® Systems).
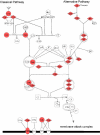
Figure 4. Pathway diagram showing the molecules involved in complement pathway and their interaction.
The diagram was modified from Ingenuity Pathway Analysis (Ingenuity® Systems).
Figure 5. Active leukocytes recruitment in aged RPE/choroid.
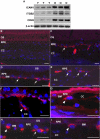
(A) Comparison of protein levels of ICAM1, ITGB2 and CD45 in RPE/choroid of young (Y, n = 3) and old (O, n = 3) mice by immunoblot. ICAM1, ITGB2 and CD45 are all increased in RPE/choroids from old animals. β-actin was used as a loading control. (B–I) Localization of leukocytes in young and old RPE/choroid. Leukocytes are labeled with leukocyte common antigen, CD45 (red). (B) In the young RPE/choroid, there are no leukocytes attached to Bruch's membrane or in the RPE layer. (C–I) Leukocytes in the old RPE/choroid. There are many leukocytes attached to Bruch's membrane in the RPE/choroid in old animals (C–E, arrows). Note the leukocyte that is attaching to the endothelial surface of the choroidal capillary (F, arrow heads), the leukocyte that migrated from the vessel to the local tissue (G, arrow heads), and the leukocyte passing through Bruch's membrane (H, arrow heads). The leukocyte attaching to Bruch's membrane has a lobated nucleus, indicating a polymorphonuclear leukocyte (I, arrow heads). OS, outer segment; RPE, retinal pigment epithelium; CC, choroidal capillaries; BM, Bruch's membrane. Scale bar = 20 µm.
Figure 6. Activation of complement pathway in RPE/choroid of old animals.
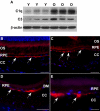
(A) Comparison of protein levels of C1q and C3 in RPE/choroid of young (Y, n = 3) and old (O, n = 3) mice by immunoblot. Both C1q and C3 are increased in aged RPE/choroids. β-actin was used as a loading control. (B–E) C3 deposition (red) in RPE/choroids. (B) C3 staining shows a thin and continuous line at Bruch's membrane in young animals (arrows). (C–E) In old animals, C3 deposition shows large and discontinuous clumps at Bruch's membrane (arrows). Scale bar = 50 µm (B–D) or 20 µm (E).
Figure 7. Increase of Ccl2 in RPE/choroid of old animals.
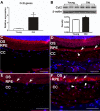
(A) Comparison of Ccl2 gene expression in RPE/choroid between young and old mice. The expression level of Ccl2 is significantly increased in aged RPE/choroid (*p<0.01, n = 4). (B) Comparison of protein levels of Ccl2 in RPE/choroid of young (Y, n = 3) and old (O, n = 3) mice by immunoblot. Ccl2 is increased in aged RPE/choroids. Densitometric measurements confirm a significant increase in Ccl2 protein level in aged RPE/choroid (*p<0.01, n = 3). (C–F) Ccl2 distribution in RPE/choroid. Ccl2 is expressed in RPE cells (arrows) and endothelial cells (arrowheads). There is weak labeling for Ccl2 in the young RPE/choroid (C, E); and the amount of Ccl2 appears increased in old animals, especially in the choroid (D, F). Scale bar = 50 µm.
Similar articles
- Comparison of Mouse and Human Retinal Pigment Epithelium Gene Expression Profiles: Potential Implications for Age-Related Macular Degeneration.
Bennis A, Gorgels TG, Ten Brink JB, van der Spek PJ, Bossers K, Heine VM, Bergen AA. Bennis A, et al. PLoS One. 2015 Oct 30;10(10):e0141597. doi: 10.1371/journal.pone.0141597. eCollection 2015. PLoS One. 2015. PMID: 26517551 Free PMC article. - Age-related changes in the transcriptional profile of mouse RPE/choroid.
Ida H, Boylan SA, Weigel AL, Hjelmeland LM. Ida H, et al. Physiol Genomics. 2003 Nov 11;15(3):258-62. doi: 10.1152/physiolgenomics.00126.2003. Physiol Genomics. 2003. PMID: 14519767 - An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration.
Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. Hageman GS, et al. Prog Retin Eye Res. 2001 Nov;20(6):705-32. doi: 10.1016/s1350-9462(01)00010-6. Prog Retin Eye Res. 2001. PMID: 11587915 Review.
Cited by
- Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal.
Kohno H, Chen Y, Kevany BM, Pearlman E, Miyagi M, Maeda T, Palczewski K, Maeda A. Kohno H, et al. J Biol Chem. 2013 May 24;288(21):15326-41. doi: 10.1074/jbc.M112.448712. Epub 2013 Apr 9. J Biol Chem. 2013. PMID: 23572532 Free PMC article. - Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration.
Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Wang AL, et al. PLoS One. 2009;4(1):e4160. doi: 10.1371/journal.pone.0004160. Epub 2009 Jan 8. PLoS One. 2009. PMID: 19129916 Free PMC article. - An allosteric interleukin-1 receptor modulator mitigates inflammation and photoreceptor toxicity in a model of retinal degeneration.
Dabouz R, Cheng CWH, Abram P, Omri S, Cagnone G, Sawmy KV, Joyal JS, Desjarlais M, Olson D, Weil AG, Lubell W, Rivera JC, Chemtob S. Dabouz R, et al. J Neuroinflammation. 2020 Nov 27;17(1):359. doi: 10.1186/s12974-020-02032-8. J Neuroinflammation. 2020. PMID: 33246504 Free PMC article. - AZGP1 Attenuates Subretinal Fibrosis and Inhibits Epithelial-Mesenchymal Transition by Blocking the PI3K/AKT Signaling Pathway.
Yang Y, Shen J, Li Y, Chen X, Liu G, Lu P. Yang Y, et al. Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):83. doi: 10.1167/iovs.66.4.83. Invest Ophthalmol Vis Sci. 2025. PMID: 40305469 Free PMC article. - Retinal phagocytes in age-related macular degeneration.
Kim SY. Kim SY. Macrophage (Houst). 2015;2(1):e698. doi: 10.14800/macrophage.698. Macrophage (Houst). 2015. PMID: 26052551 Free PMC article.
References
- Burke D, Hickie I, Breakspear M, Gotz J. Possibilities for the prevention and treatment of cognitive impairment and dementia. Br J Psychiatry. 2007;190:371–372. - PubMed
- Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–1352. - PubMed
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. - PubMed
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. - PubMed
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases