The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology - PubMed (original) (raw)
The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology
Grazia Devigili et al. Brain. 2008 Jul.
Abstract
Small fibre neuropathy (SFN), a condition dominated by neuropathic pain, is frequently encountered in clinical practise either as prevalent manifestation of more diffuse neuropathy or distinct nosologic entity. Aetiology of SFN includes pre-diabetes status and immune-mediated diseases, though it remains frequently unknown. Due to their physiologic characteristics, small nerve fibres cannot be investigated by routine electrophysiological tests, making the diagnosis particularly difficult. Quantitative sensory testing (QST) to assess the psychophysical thresholds for cold and warm sensations and skin biopsy with quantification of somatic intraepidermal nerve fibres (IENF) have been used to determine the damage to small nerve fibres. Nevertheless, the diagnostic criteria for SFN have not been defined yet and a 'gold standard' for clinical practise and research is not available. We screened 486 patients referred to our institutions and collected 124 patients with sensory neuropathy. Among them, we identified 67 patients with pure SFN using a new diagnostic 'gold standard', based on the presence of at least two abnormal results at clinical, QST and skin biopsy examination. The diagnosis of SFN was achieved by abnormal clinical and skin biopsy findings in 43.3% of patients, abnormal skin biopsy and QST findings in 37.3% of patients, abnormal clinical and QST findings in 11.9% of patients, whereas 7.5% patients had abnormal results at all the examinations. Skin biopsy showed a diagnostic efficiency of 88.4%, clinical examination of 54.6% and QST of 46.9%. Receiver operating characteristic curve analysis confirmed the significantly higher performance of skin biopsy comparing with QST. However, we found a significant inverse correlation between IENF density and both cold and warm thresholds at the leg. Clinical examination revealed pinprick and thermal hypoesthesia in about 50% patients, and signs of peripheral vascular autonomic dysfunction in about 70% of patients. Spontaneous pain dominated the clinical picture in most SFN patients. Neuropathic pain intensity was more severe in patients with SFN than in patients with large or mixed fibre neuropathy, but there was no significant correlation with IENF density. The aetiology of SFN was initially unknown in 41.8% of patients and at 2-year follow-up a potential cause could be determined in 25% of them. Over the same period, 13% of SFN patients showed the involvement of large nerve fibres, whereas in 45.6% of them the clinical picture did not change. Spontaneous remission of neuropathic pain occurred in 10.9% of SFN patients, while it worsened in 30.4% of them.
Figures
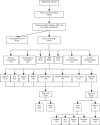
Fig. 1
Flow diagram showing the various categories of sensory neuropathies diagnosed after clinical examination, nerve conduction studies, QST and skin biopsy. The aetiology of SFN at first observation and at 2-year follow-up is detailed.
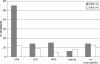
Fig. 2
Correlation between intensity of neuropathic pain and type of neuropathy in 124 patients. ‘Others’ include mononeuropathies and sensory neuronopathies. ‘No neuropathy’ include patients in whom the diagnosis of neuropathy was ruled out.
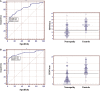
Fig. 3
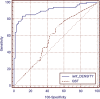
ROC analysis of IENF density at the proximal thigh (A) and the distal leg (B) in 110 patients with painful sensory neuropathy and 47 healthy controls. At the proximal thigh, the cut-off value was 12.8 IENF/mm (SE = 0.035, area under the curve = 0.825 and 95% CI = 0.756–0.882). At the distal leg, the cut-off value was 7.63 IENF/mm (SE = 0.026, area under the curve = 0.906 and 95% CI = 0.849–0.947).
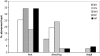
Fig. 4
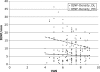
QST at dorsal foot, distal leg and proximal thigh in healthy controls (CTRL) and patients with SFN, MFN and LFN. Box plots represent the median value with 25th and 75th percentiles.
Fig. 5
Distribution of abnormal QST findings in 67 patients with SFN. ES = errata sensation; CS = cold sensation; WS = warm sensation; CP = cold pain; HP = heat pain.
Fig. 6
ROC analysis in 67 patients with SFN comparing the diagnostic yield of skin biopsy with quantification of IENF and QST at the distal leg. Area under the ROC was 0.904 for IENF density (SE = 0.027; 95% CI = 0.841–0.947) and was 0.576 for QST (SE = 0.049; 95% CI = 0.488–0.660). Difference between areas was 0.328 (SE = 0.053; 95% CI = 0.225–0.431; P < 0.001).
Fig. 7
Correlation between intensity of pain measured by the VAS and the linear innervation density of the epidermis (IENF/mm) at proximal thigh (Pth) and distal leg (Dl) in 67 patients with SFN. No significant correlation was found.
Fig. 8
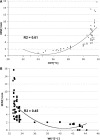
Correlation analysis of IENF density (IENF/mm), (CDT; A) and (WDT; B) at the distal leg in patients with SFN. Data did not show a normal distribution. Non-parametric Mann−Whitney U-test demonstrated the significant correlation between IENF/mm and both CDT (P < 0.0001) and WDT (P < 0.0001) at the distal leg.
Comment in
- Pitfalls of diagnostic criteria for small fiber neuropathy.
Botez SA, Herrmann DN. Botez SA, et al. Nat Clin Pract Neurol. 2008 Nov;4(11):586-7. doi: 10.1038/ncpneuro0920. Epub 2008 Oct 7. Nat Clin Pract Neurol. 2008. PMID: 18839004
Similar articles
- Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy.
Løseth S, Lindal S, Stålberg E, Mellgren SI. Løseth S, et al. Eur J Neurol. 2006 Feb;13(2):105-11. doi: 10.1111/j.1468-1331.2006.01232.x. Eur J Neurol. 2006. PMID: 16490039 - Small-fibre involvement in diabetic patients with neuropathic foot pain.
Vlckova-Moravcova E, Bednarik J, Belobradkova J, Sommer C. Vlckova-Moravcova E, et al. Diabet Med. 2008 Jun;25(6):692-9. doi: 10.1111/j.1464-5491.2008.02446.x. Diabet Med. 2008. PMID: 18544107 - The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy.
Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Loavenbruck A, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M. Provitera V, et al. Eur J Neurol. 2018 Jun;25(6):848-853. doi: 10.1111/ene.13608. Epub 2018 Apr 6. Eur J Neurol. 2018. PMID: 29493845 - [Small fibre neuropathy: knowledge is power].
Hoeijmakers JG, Bakkers M, Blom EW, Drenth JP, Merkies IS, Faber CG. Hoeijmakers JG, et al. Ned Tijdschr Geneeskd. 2012;156(7):A4224. Ned Tijdschr Geneeskd. 2012. PMID: 22333400 Review. Dutch.
Cited by
- Intra- and interfamily phenotypic diversity in pain syndromes associated with a gain-of-function variant of NaV1.7.
Estacion M, Han C, Choi JS, Hoeijmakers JG, Lauria G, Drenth JP, Gerrits MM, Dib-Hajj SD, Faber CG, Merkies IS, Waxman SG. Estacion M, et al. Mol Pain. 2011 Dec 2;7:92. doi: 10.1186/1744-8069-7-92. Mol Pain. 2011. PMID: 22136189 Free PMC article. - Acute monophasic erythromelalgia pain in five children diagnosed as small-fiber neuropathy.
Faignart N, Nguyen K, Soroken C, Poloni C, Downs HM, Laubscher B, Korff C, Oaklander AL, Roulet Perez E. Faignart N, et al. Eur J Paediatr Neurol. 2020 Sep;28:198-204. doi: 10.1016/j.ejpn.2020.06.004. Epub 2020 Jul 7. Eur J Paediatr Neurol. 2020. PMID: 32723684 Free PMC article. - Distinguishing fibromyalgia syndrome from small fiber neuropathy: a clinical guide.
Jänsch S, Evdokimov D, Egenolf N, Meyer Zu Altenschildesche C, Kreß L, Üçeyler N. Jänsch S, et al. Pain Rep. 2024 Jan 24;9(1):e1136. doi: 10.1097/PR9.0000000000001136. eCollection 2024 Jan. Pain Rep. 2024. PMID: 38283649 Free PMC article. - Comparison of diabetic and idiopathic sensory polyneuropathies with respect to nerve fibre affection and risk factors.
Itani M, Gylfadottir S, Krøigård T, Gaist L, Holbech JV, Kristensen AG, Karlsson P, Möller S, Tankisi H, Gaist D, Jensen TS, Finnerup NB, Sindrup SH. Itani M, et al. BMJ Neurol Open. 2022 Mar 14;4(1):e000247. doi: 10.1136/bmjno-2021-000247. eCollection 2022. BMJ Neurol Open. 2022. PMID: 35360409 Free PMC article. - Patient-derived in vitro skin models for investigation of small fiber pathology.
Karl F, Wußmann M, Kreß L, Malzacher T, Fey P, Groeber-Becker F, Üçeyler N. Karl F, et al. Ann Clin Transl Neurol. 2019 Sep;6(9):1797-1806. doi: 10.1002/acn3.50871. Epub 2019 Aug 28. Ann Clin Transl Neurol. 2019. PMID: 31464071 Free PMC article.
References
- Al-Shekhlee A, Chelimsky TC, Preston DC. Review: small-fiber neuropathy. Neurologist. 2002;8:237–53. - PubMed
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. - PubMed
- Baron R. Mechanisms of disease: neuropathic pain- -a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. - PubMed
- Brannagan TH, 3rd, Hays AP, Chin SS, Sander HW, Chin RL, Magda P, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol. 2005;62:1574–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous