Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development - PubMed (original) (raw)
Comparative Study
Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development
Takuji Iwasato et al. J Neurosci. 2008.
Abstract
Experimental evidence from mutant or genetically altered mice indicates that the formation of barrels and the proper maturation of thalamocortical (TC) synapses in the primary somatosensory (barrel) cortex depend on mechanisms mediated by neural activity. Type 1 adenylyl cyclase (AC1), which catalyzes the formation of cAMP, is stimulated by increases in intracellular Ca(2+) levels in an activity-dependent manner. The AC1 mutant mouse, barrelless (brl), lacks typical barrel cytoarchitecture, and displays presynaptic and postsynaptic functional defects at TC synapses. However, because AC1 is expressed throughout the trigeminal pathway, the barrel cortex phenotype of brl mice may be a consequence of AC1 disruption in cortical or subcortical regions. To examine the role of cortical AC1 in the development of morphological barrels and TC synapses, we generated cortex-specific AC1 knock-out (CxAC1KO) mice. We found that neurons in layer IV form grossly normal barrels and TC axons fill barrel hollows in CxAC1KO mice. In addition, whisker lesion-induced critical period plasticity was not impaired in these mice. However, we found quantitative reductions in the quality of cortical barrel cytoarchitecture and dendritic asymmetry of layer IV barrel neurons in CxAC1KO mice. Electrophysiologically, CxAC1KO mice have deficits in the postsynaptic but not in the presynaptic maturation of TC synapses. These results suggest that activity-dependent postsynaptic AC1-cAMP signaling is required for functional maturation of TC synapses and the development of normal barrel cortex cytoarchitecture. They also suggest that the formation of the gross morphological features of barrels is independent of postsynaptic AC1 in the barrel cortex.
Figures
Figure 1.
Cre-mediated recombination on the AC1-flox allele produces AC1-null allele. A, Schematics of AC1flox and AC1Δ (= null or −) alleles. AC1Δ allele was generated by expressing Cre recombinase in the germline of AC1flox/+ mice, in which exon 1 and the putative promoter of the AC1 gene are flanked by two loxP sites. B, RT-PCR using primers specific for exons downstream of the floxed region (shown in A) detects AC1 mRNA expression in AC1flox/flox control brains (3, 4) but not in AC1Δ/Δ brains (1, 2), demonstrating that the AC1-flox allele is functional. m, Size markers. C–F, Flattened cortices stained for CO (C, D) or Nissl (E, F) demonstrate that AC1Δ/Δ mice (D, F) completely lack the cortical barrel pattern, unlike control (wild-type) mice (C, E). G, H, Barreloids in VB thalamus of AC1Δ/Δ mice (H) are not as distinct as those of (AC1+/Δ) control mice (G). I, J, Both control (AC1+/Δ) and AC1Δ/Δ mice have normal barrelettes in the brainstem principal nucleus. The whisker-related patterns in AC1Δ/Δ mice throughout the trigeminal pathway are similar to those reported in barrelless (brl) and AC1−/− mice (Welker et al., 1996; Abdel-Majid et al., 1998), further confirming that the AC1flox allele is functional. Scale bar: C–F, 500 μm; G, H, 250 μm; I, J, 125 μm.
Figure 2.
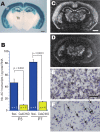
CxAC1KO mice generated using Cre expression driven by Emx1 promoter. A, LacZ staining of 400-μm-thick coronal sections from progeny of Emx1-Cre (KΔN) mice crossed with CAG-CAT-Z reporter mice demonstrates that Cre-mediated recombination is specific to neocortex and hippocampus from embryonic stages (data not shown) to adulthood. For more information, see Iwasato et al. (2000, 2004). SI, Barrel cortex; Hip, hippocampus; Th, thalamus. B, Quantitative RT-PCR of AC1flox/− and CxAC1KO mice barrel cortex at P3 and P7. Number of AC1 transcripts per picogram of total RNA for P1 flox/− (n = 5), P1 CxAC1KO (n = 4), P7 flox/− (n = 4), and P7 CxAC1KO (n = 4) are as follows (mean ± SEM): 46.86 ± 6.37, 9.36 ± 1.42, 81.96 ± 4.43, and 14.83 ± 1.09; Student's t test. C, D, In situ hybridization of AC1 mRNA in coronal sections of P7 brains of AC1flox/− (C, E) and CxAC1KO (D, F) mice at P7. A few AC1-expressing neurons (F, arrows), presumably GABAergic interneurons, are observed in CxAC1KO cortex. Scale bars: A, C, D, 1 mm; E, F, 50 μm.
Figure 3.
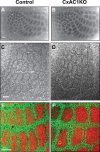
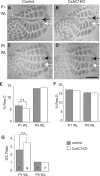
Grossly normal cortical barrel map in CxAC1KO mice. A, B, Tangential sections from P29 control (AC1flox/−) (A) and CxAC1KO (B) mice stained with CO. Whisker barrel patterns are indistinguishable between genotypes. C, D, Nissl-stained tangential sections from P24 control (AC1flox/−) (C) and CxAC1KO (D) mice. As in control mice, layer IV neurons in CxAC1KO mice organize into a grossly normal barrel “wall” and “hollow” pattern characteristic of rodent somatosensory cortex. E, F, Tangential sections from P10 control (AC1flox/−) (E) and CxAC1KO (F) mice immunostained with anti-vGlut2 antibody in red and anti-NeuN in green, which selectively label TC afferents and neuronal cell bodies, respectively. The barrel pattern is qualitatively similar in the two genotypes, showing that the gross presynaptic and postsynaptic patterning of barrels in CxAC1KO mice is not impaired. However, a quantitative analysis of the patterning of layer IV neurons into barrel walls and hollows reveals a small but statistically significant decrease in the wall-to-hollow ratio in the CxAC1KO mice (1.28 ± 0.032; n = 11) compared with AC1flox/− control mice (1.47 ± 0.035; n = 8; p < 0.01, Student's t test). Scale bars, 100 μm.
Figure 4.
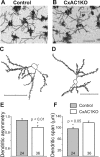
Dendritic asymmetry of layer IV spiny stellate neurons in CxAC1KO mice is reduced compared with littermate controls. A–D, Examples of Golgi-stained layer IV spiny stellate neurons in the barrel cortex (A, B) and their computer-aided reconstructions (C, D) in control (AC1flox/−) (A, C) and CxAC1KO (B, D) mice at P29. E, CxAC1KO mice have significantly lower (*p < 0.01, Student's t test) dendritic asymmetry compared with littermate controls. Dendritic asymmetry, which must be ≥0.5, is defined as the ratio of the dendritic length in the hemisphere with the greatest density of dendrites relative to the total dendritic length. Error bars indicate SEM. F, Dendritic field span, the greatest distance between the most distal dendrite tips of a particular layer IV spiny stellate neuron, is significantly greater (*p < 0.05, Student's t test) in CxAC1KO mice compared with littermate control mice. Scale bars, 50 μm.
Figure 5.
Lesion-induced plasticity is not altered in CxAC1KO mice. A, B, 5HTT immunohistochemistry of tangential sections from barrel cortex shows that C row whisker lesioned at P1 (P1WL) induces robust shrinkage of row C (black arrows) and expansion of neighboring rows (row D, white arrows) in TC axons of both CxAC1KO (B) and their littermate control (AC1flox/−) mice (A). C, D, The critical period for this map plasticity ends by P5 in both CxAC1KO (D) and littermate control mice (C). E–G, Quantification of lesion-induced plasticity in control and CxAC1KO mice. Analyses of percentage row C (E) and row D (F) areas normalized with respect to area of the entire large whisker representation are similar between control and CxAC1KO mice. The D/C ratio calculated by dividing the row D area by the row C area provides a plasticity index (Schlaggar et al., 1993; Datwani et al., 2002b). The D/C ratio analysis reveals no difference in TC axon map plasticity between genotypes (G). Error bars indicate SEM; Student's t test. Scale bar, 500 μm.
Figure 6.
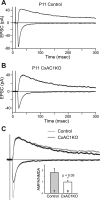
AMPA/NMDA current ratio is reduced in CxAC1KO mice. A, B, Sample whole-cell voltage-clamp measurements of AMPA receptor-mediated and NMDA receptor-mediated TC EPSCs in a P11 littermate control (A), and P11 CxAC1KO (B) mouse. C, Overlay of the responses in A and B scaled so that the NMDA receptor currents are the same amplitude. Note the AMPA receptor-mediated EPSC in CxAC1KO animal is small compared with the littermate control. Inset, Summary quantification of AMPA/NMDA current ratios of control (AC1flox/−) and CxAC1KO mice at P9–P11. The AMPA/NMDA current ratio of CxAC1KO mice is significantly smaller than that of control mice (*p < 0.05; t test). Error bars indicate SEM.
Figure 7.
AMPA receptor mini-EPSCs are smaller at CxAC1KO TC synapses. A, B, Sample traces of evoked AMPA receptor-mediated current responses in Ca2+-ACSF (gray) and Sr2+-ACSF (black) from recordings in P9 CxAC1KO (B) and littermate control (AC1flox/−) (A) neurons. In the presence of Sr2+-ACSF, responses to evoked synchronous release is lower and quantal events (evoked mini-EPSCs) in response to asynchronous release appear. The amplitudes of these quantal events are smaller on average in CxAC1KO mice. C, Average frequency histogram of evoked mini-EPSCs. The CxAC1KO histogram (white bars) peaks at a smaller amplitude than littermate controls (gray bars), and large amplitude events are almost absent in CxAC1KO mice. Inset, Mean evoked mini-EPSC amplitude is significantly lower (*p < 0.05, t test) for CxAC1KO mice (white) with respect to littermate controls (gray). Error bars indicate SEM. D, The cumulative probability distribution in CxAC1KO animals (white) also shows a shift toward smaller amplitudes compared with littermate controls (gray).
Figure 8.
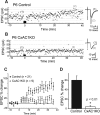
LTP is defective at CxAC1KO TC synapses. A, B, Example whole-cell voltage-clamp recordings from P6 littermate control (AC1flox/−) (A) and P6 CxAC1KO (B) neurons show that TC AMPA response in control but not CxAC1KO layer IV neurons can be potentiated using an LTP “pairing” protocol. Traces on the right (average of 20 sweeps) from before (1) and 35 min after (2) pairing show significant potentiation in control but not in CxAC1KO neurons. C, Summary graph of pairing experiments showing the EPSC percentage change for CxAC1KO mice (open circles) and controls (AC1flox/−, Emx1-Cre;AC1+/−, AC1+/−, and AC1flox/+) (filled circles). Control TC synapses reveal obvious potentiation, whereas CxAC1KOs cannot potentiate. Error bars indicate SEM. D, Summary histogram of EPSC percentage change from the average of 20 sweeps starting at 35 min after pairing for control and CxAC1KO TC synapses. CxAC1KO TC synapses show almost no potentiation and their EPSC percentage change is significantly lower (*p < 0.01, Student's t test) than that of control mice.
Figure 9.
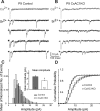
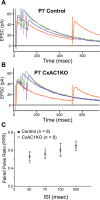
PPR at CxAC1KO TC synapses is similar to littermate controls. A, B, Average example EPSC responses of a P7 CxAC1KO (B) and a P7 littermate control (AC1flox/−) (A) cell to paired stimuli at ISIs 50 ms (blue), 75 ms (green), 100 ms (purple), and 500 ms (orange) reveal similar PPRs. C, Summary plot for average PPRs at ISIs at 50, 75, 100, and 500 ms are not different between CxAC1KO (open circles) and littermate controls (black circles) at P5–P7, suggesting that Pr at CxAC1KO TC synapses is not defective. Error bars indicate SEM.
Figure 10.
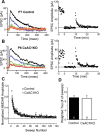
CxAC1KO TC synapses exhibit no difference in Pr. A, B, Sample traces of a P6 CxAC1KO (B) and a P7 littermate control (AC1flox/−) (A) taken at progressively later times after MK801 wash and the corresponding experiments with the NMDA receptor response amplitude versus time (right). A baseline NMDA receptor current is acquired before turning the stimulation off to wash on MK801 (10 μ
m
). After resuming stimulation, the NMDA receptor current amplitude decreases steadily with a rate that is proportional to the probability of release. Color of the sample traces (A, B) corresponds to the points colored on the amplitude plot on the right panel. C, Comparison of littermate control and CxAC1KO mice at P5–P7 reveals no difference in the rate of NMDA receptor block by MK801, further indicating that the Pr is not different between CxAC1KO and control TC synapses. Error bars indicate SEM. D, Weighted time constants (tau) measured by fitting a double-exponential curve to the NMDA receptor amplitude decay over time are not different between genotypes.
Similar articles
- Region-Specific Disruption of Adenylate Cyclase Type 1 Gene Differentially Affects Somatosensorimotor Behaviors in Mice(1,2,3).
Arakawa H, Akkentli F, Erzurumlu RS. Arakawa H, et al. eNeuro. 2014 Nov 12;1(1):ENEURO.0007-14.2014. doi: 10.1523/ENEURO.0007-14.2014. eCollection 2014 Nov-Dec. eNeuro. 2014. PMID: 26464960 Free PMC article. - Thalamic adenylyl cyclase 1 is required for barrel formation in the somatosensory cortex.
Suzuki A, Lee LJ, Hayashi Y, Muglia L, Itohara S, Erzurumlu RS, Iwasato T. Suzuki A, et al. Neuroscience. 2015 Apr 2;290:518-29. doi: 10.1016/j.neuroscience.2015.01.043. Epub 2015 Jan 30. Neuroscience. 2015. PMID: 25644422 Free PMC article. - Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling.
Inan M, Lu HC, Albright MJ, She WC, Crair MC. Inan M, et al. J Neurosci. 2006 Apr 19;26(16):4338-49. doi: 10.1523/JNEUROSCI.3745-05.2006. J Neurosci. 2006. PMID: 16624954 Free PMC article. - Spike timing and synaptic dynamics at the awake thalamocortical synapse.
Swadlow HA, Bezdudnaya T, Gusev AG. Swadlow HA, et al. Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
Cited by
- How the Barrel Cortex Became a Working Model for Developmental Plasticity: A Historical Perspective.
Erzurumlu RS, Gaspar P. Erzurumlu RS, et al. J Neurosci. 2020 Aug 19;40(34):6460-6473. doi: 10.1523/JNEUROSCI.0582-20.2020. J Neurosci. 2020. PMID: 32817388 Free PMC article. - Region-Specific Disruption of Adenylate Cyclase Type 1 Gene Differentially Affects Somatosensorimotor Behaviors in Mice(1,2,3).
Arakawa H, Akkentli F, Erzurumlu RS. Arakawa H, et al. eNeuro. 2014 Nov 12;1(1):ENEURO.0007-14.2014. doi: 10.1523/ENEURO.0007-14.2014. eCollection 2014 Nov-Dec. eNeuro. 2014. PMID: 26464960 Free PMC article. - Supernova: A Versatile Vector System for Single-Cell Labeling and Gene Function Studies in vivo.
Luo W, Mizuno H, Iwata R, Nakazawa S, Yasuda K, Itohara S, Iwasato T. Luo W, et al. Sci Rep. 2016 Oct 24;6:35747. doi: 10.1038/srep35747. Sci Rep. 2016. PMID: 27775045 Free PMC article. - Epigenetic modulation rescues neurodevelopmental deficits in Syngap1+/- mice.
Singh AK, Joshi I, Reddy NMN, Purushotham SS, Eswaramoorthy M, Vasudevan M, Banerjee S, Clement JP, Kundu TK. Singh AK, et al. Aging Cell. 2025 Mar;24(3):e14408. doi: 10.1111/acel.14408. Epub 2025 Jan 29. Aging Cell. 2025. PMID: 39878322 Free PMC article. - NMDA Receptor Enhances Correlation of Spontaneous Activity in Neonatal Barrel Cortex.
Mizuno H, Rao MS, Mizuno H, Sato T, Nakazawa S, Iwasato T. Mizuno H, et al. J Neurosci. 2021 Feb 10;41(6):1207-1217. doi: 10.1523/JNEUROSCI.0527-20.2020. Epub 2020 Dec 28. J Neurosci. 2021. PMID: 33372060 Free PMC article.
References
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. - PubMed
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. - PubMed
- Bloodgood BL, Sabatini BL. Ca2+ signaling in dendritic spines. Curr Opin Neurobiol. 2007;17:345–351. - PubMed
- Chan CH, Godinho LN, Thomaidou D, Tan SS, Gulisano M, Parnavelas JG. Emx1 is a marker for pyramidal neurons of the cerebral cortex. Cereb Cortex. 2001;11:1191–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 NS007224/NS/NINDS NIH HHS/United States
- R01 MH62639/MH/NIMH NIH HHS/United States
- R01 NS039050/NS/NINDS NIH HHS/United States
- R01 MH062639/MH/NIMH NIH HHS/United States
- R01 EY015788/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous