Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I - PubMed (original) (raw)
Comparative Study
Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I
Eric S Guire et al. J Neurosci. 2008.
Abstract
Ca(2+)-permeable AMPA receptors (CP-AMPARs) at central glutamatergic synapses are of special interest because of their unique biophysical and signaling properties that contribute to synaptic plasticity and their roles in multiple neuropathologies. However, intracellular signaling pathways that recruit synaptic CP-AMPARs are unknown, and involvement of CP-AMPARs in hippocampal region CA1 synaptic plasticity is controversial. Here, we report that intracellular infusion of active CaM-kinase I (CaMKI) into cultured hippocampal neurons enhances miniature EPSC amplitude because of recruitment of CP-AMPARs, likely from an extrasynaptic pool. The ability of CaMKI, which regulates the actin cytoskeleton, to recruit synaptic CP-AMPARs was blocked by inhibiting actin polymerization with latrunculin A. CaMK regulation of CP-AMPARs was also confirmed in hippocampal slices. CA1 long-term potentiation (LTP) after theta bursts, but not high-frequency tetani, produced a rapid, transient expression of synaptic CP-AMPARs that facilitated LTP. This component of TBS LTP was blocked by inhibition of CaM-kinase kinase (CaMKK), the upstream activator of CaMKI. Our calculations show that adding CP-AMPARs numbering <5% of existing synaptic AMPARs is sufficient to account for the potentiation observed in LTP. Thus, synaptic expression of CP-AMPARs is a very efficient mechanism for rapid enhancement of synaptic strength that depends on CaMKK/CaMKI signaling, actin dynamics, and the pattern of synaptic activity used to induce CA1 LTP.
Figures
Figure 1.
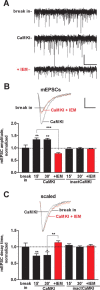
CaMKI activity promotes synaptic CP-AMPAR expression. A, Representative traces of spontaneous AMPAR mEPSCs from a single experiment during the initial 5 min of infusion of activated CaMKI (500 n
m
) (break in, top trace). After 15 min of CaMKI infusion, the amplitude of mEPSCs was significantly potentiated (middle trace). This potentiation was reversed by subsequent treatment for 15 min with a selective inhibitor of CP-AMPARs, IEM-1460 (30 μ
m
, bottom trace). Calibration: 10 pA, 2 s. B, C, Infusion of activated CaMKI (500 n
m
) into cultured hippocampal pyramidal cells increased AMPAR mEPSC amplitude (B) and decreased decay time (C, 90–10% decay time) compared with break in (n = 7). In contrast, infusion of inactive CaMKI (heat inactivated; see Fig. 2_F_) had no effect on the amplitude and decay time of mEPSCs (n = 4). Both effects of active CaMKI were reversed by bath application of IEM (30 μ
m
) beginning 15 min after break in (n = 4–5), whereas IEM had no significant effects on mEPSCs recorded from cells infused with inactive CaMKI (n = 3). Insets, Representative traces of mEPSCs showing responses during the initial 5 min (break in), after 15 min infusion of active CaMKI, and after an additional 15 min with IEM (red traces). Calibration: 5 pA, 5 ms. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars indicate SEM.
Figure 2.
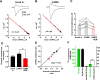
CaMKI increases channel conductance of synaptic AMPARs. A, B, Representative nonstationary fluctuation analyses for mEPSCs recorded in the same cell after break in (A) and after 15 min of caCaMKI infusion (B). Insets, Bottom traces are averages of 158 and 199 mEPSCs for A and B, respectively; top traces are the corresponding variances. Calibrations are the same for A and B. Graphs are variance–mEPSC relationships for traces shown in insets. Red lines are fittings by the equation described in Materials and Methods. Estimated channel conductances are indicated as γ. C, Changes in single-channel conductance in individual cells (open circles) after break in, infusion of CaMKI alone for 15 min (n = 8), or followed by another 15 min of CaMKI in the presence of 30 μ
m
IEM-1460 (n = 4) (see also Fig. 1). Error bars show mean values of γ and SEMs. D, Relative changes in channel conductance for cells in C with break in values normalized to 1. E, Changes in mEPSC amplitude as a function of channel conductance. For each cell, ratios of mean mEPSCs for CaMKI infusion and break in (n = 8; black circles), and IEM application with CaMKI infusion (n = 4; red circles), were plotted against corresponding ratios of channel conductances. Points were fitted by a linear function: (mEPSC amplitude ratio) = 0.12 + 0.93 × (channel conductance ratio). F, CaMKI does not phosphorylate the C terminus of GluR1. CaMKII and CaMKI (activated by CaMKK) were assayed for 32P incorporation into the C terminus of GluR1 (Barria et al., 1997) and the synthetic peptide substrate syntide-2. CPM, Counts per minute. Note the different scales for CaMKI and CaMKII and that heat-inactived CaMKI (used in Fig. 1) was catalytically inactive. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 3.
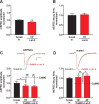
Actin polymerization is required for CaMKI-mediated synaptic expression of CP-AMPARs. A, B, Under basal conditions, intracellular infusion of latrunculin A (2 μ
m
, n = 7) to inhibit actin polymerization resulted in a small inhibition of mEPSC amplitude (A) but not decay kinetics (B). C, D, Coinfusion of latrunculin A (Lat.A) with activated CaMKI blocked both the increases in mEPSC amplitude (C, n = 7 for 15 min and n = 3 for 30 min) and acceleration in kinetics (D) caused by infused CaMKI alone (effects of CaMKI alone are indicated by dashed lines and taken from Fig. 1). Insets, Averaged mEPSCs recorded during first 2 min (break in) and after 15 min of coinfusion (caCaMKI+latrunculin). Calibration: 1 pA, 5 ms. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars indicate SEM.
Figure 4.
Extrasynaptic CP-AMPARs are expressed on dendrites under basal conditions. The experiments in this figure were performed without infusion of kinase. A, AMPA (0.5 m
m
) was briefly (10 ms) applied to a dendrite 100 micron from the soma, and AMPA currents were measured at different holding potentials (from −80 to 80 mV, step 40 mV). B, Current–voltage (I–V) relationships for dendritic AMPA currents (n = 4, SEs are less than the size of symbols). The dashed line indicates a linear I–V extrapolated from points at negative potentials. A significant rectification of the I–V curve at positive potentials indicates that CP-AMPARs are present on the dendritic surface. C, In the presence of IEM-1460 (100 μ
m
), dendritic AMPA-currents were significantly inhibited (the same cell as in A), independently confirming the presence of CP-AMPARs. D, In contrast, mEPSCs (n = 3) were not affected by IEM-1460 (see also Fig. 1_B_) when measured in the same cells as AMPA currents, indicating that CP-AMPARs are not in synapses. *p < 0.05. Error bars indicate SEM.
Figure 5.
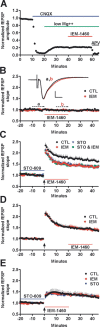
CaMKK regulates transient synaptic CP-AMPAR expression during TBS-LTP. Field recordings (fEPSPs) from area CA1 (stratum radiatum) elicited by SC-stimulation (see Materials and Methods) in acute hippocampal slices (4- to 6-week-old rat). A confirms that IEM (30 μ
m
) does not inhibit NMDARs (pharmacologically isolated with 10 μ
m
CNQX in 0.1 m
m
Mg2+), and B demonstrates that IEM (30 μ
m
) has no effect on basal synaptic transmission in naive slices (n = 6). Inset, Representative fEPSP averages before (a, black trace) and during (b, red trace). Calibration: 0.5 mV, 5 ms. C, Application of IEM immediately after TBS (Materials and Methods) suppressed LTP ∼40% compared with control (CTL). This IEM-sensitive component of LTP was absent in slices pretreated with the CaMKK inhibitor STO-609 (5 μ
m
), demonstrating rapid recruitment of synaptic CP-AMPARs downstream of CaMKK (n = 9–10). D, Application of IEM 20 min after TBS did not alter LTP expression, indicating that synaptic incorporation of CP-AMPARs is transient (n = 9). E, In contrast to TBS-LTP, HFS-LTP (see Materials and Methods) was not affected by STO-609 pretreatment or treatment with IEM immediately after HFS (n = 10).
Figure 6.
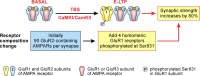
Model for potentiation of synaptic strength through CaM-kinase-mediated recruitment and regulation of GluR1 homomeric CP-AMPARs. Calculations (see Appendix) show that for a typical hippocampal SC–CA1 pyramidal cell synapse consisting of ∼90 GluR2-containing AMPARs, CaMKI-mediated recruitment of four homomeric GluR1 AMPARs and their phosphorylation on Ser831 by CaMKII is sufficient to increase synaptic strength by 80%. If these CP-AMPARs are recruited from an existing extrasynaptic pool, as indicated by our results, this process provides a very rapid and efficient mechanism for the initial synaptic potentiation (E-LTP) after induction of LTP by appropriate stimuli. Because the presence of the dominant GluR2 subunit precludes functional regulation of GluR1-containing heteromeric AMPARs because of S831 phosphorylation (Oh and Derkach, 2005), this model may also explain how CaMKI and CaMKII cooperate to maximally increase single channel conductance of AMPARs during LTP (Benke et al., 1998).
Similar articles
- Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I.
Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. Fortin DA, et al. J Neurosci. 2010 Sep 1;30(35):11565-75. doi: 10.1523/JNEUROSCI.1746-10.2010. J Neurosci. 2010. PMID: 20810878 Free PMC article. - Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II.
Asrar S, Zhou Z, Ren W, Jia Z. Asrar S, et al. PLoS One. 2009;4(2):e4339. doi: 10.1371/journal.pone.0004339. Epub 2009 Feb 3. PLoS One. 2009. PMID: 19190753 Free PMC article. - Calcium-Permeable AMPA Receptors Mediate the Induction of the Protein Kinase A-Dependent Component of Long-Term Potentiation in the Hippocampus.
Park P, Sanderson TM, Amici M, Choi SL, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL. Park P, et al. J Neurosci. 2016 Jan 13;36(2):622-31. doi: 10.1523/JNEUROSCI.3625-15.2016. J Neurosci. 2016. PMID: 26758849 Free PMC article. - How Ca2+-permeable AMPA receptors, the kinase PKA, and the phosphatase PP2B are intertwined in synaptic LTP and LTD.
Hell JW. Hell JW. Sci Signal. 2016 Apr 26;9(425):e2. doi: 10.1126/scisignal.aaf7067. Sci Signal. 2016. PMID: 27117250 Review. - The Role of Calcium-Permeable AMPARs in Long-Term Potentiation at Principal Neurons in the Rodent Hippocampus.
Park P, Kang H, Sanderson TM, Bortolotto ZA, Georgiou J, Zhuo M, Kaang BK, Collingridge GL. Park P, et al. Front Synaptic Neurosci. 2018 Nov 22;10:42. doi: 10.3389/fnsyn.2018.00042. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30524263 Free PMC article. Review.
Cited by
- Experience-dependent homeostatic synaptic plasticity in neocortex.
Whitt JL, Petrus E, Lee HK. Whitt JL, et al. Neuropharmacology. 2014 Mar;78:45-54. doi: 10.1016/j.neuropharm.2013.02.016. Epub 2013 Mar 4. Neuropharmacology. 2014. PMID: 23466332 Free PMC article. Review. - Ca-permeable AMPA receptors in homeostatic synaptic plasticity.
Lee HK. Lee HK. Front Mol Neurosci. 2012 Feb 13;5:17. doi: 10.3389/fnmol.2012.00017. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22347846 Free PMC article. - Homer1 gene products orchestrate Ca(2+)-permeable AMPA receptor distribution and LTP expression.
Rozov A, Zivkovic AR, Schwarz MK. Rozov A, et al. Front Synaptic Neurosci. 2012 Sep 27;4:4. doi: 10.3389/fnsyn.2012.00004. eCollection 2012. Front Synaptic Neurosci. 2012. PMID: 23133416 Free PMC article. - Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation.
Diógenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, Näsström T, Franquelim HG, Oliveira LM, Castanho MA, Lannfelt L, Bergström J, Ingelsson M, Quintas A, Sebastião AM, Lopes LV, Outeiro TF. Diógenes MJ, et al. J Neurosci. 2012 Aug 22;32(34):11750-62. doi: 10.1523/JNEUROSCI.0234-12.2012. J Neurosci. 2012. PMID: 22915117 Free PMC article. - Synaptic AMPA receptor plasticity and behavior.
Kessels HW, Malinow R. Kessels HW, et al. Neuron. 2009 Feb 12;61(3):340-50. doi: 10.1016/j.neuron.2009.01.015. Neuron. 2009. PMID: 19217372 Free PMC article. Review.
References
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997a;272:32727–32730. - PubMed
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997b;276:2042–2045. - PubMed
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS027037/NS/NINDS NIH HHS/United States
- R01 NS027037-18/NS/NINDS NIH HHS/United States
- R01 NS027037-19/NS/NINDS NIH HHS/United States
- R01 NS27037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous