Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1 - PubMed (original) (raw)
. 2008 Jul 9;27(13):1886-95.
doi: 10.1038/emboj.2008.113. Epub 2008 Jun 5.
Jordi Guiu, Cristina Ruiz-Herguido, M Eugenia López, Julia Inglés-Esteve, Lluis Riera, Alex Tipping, Tariq Enver, Elaine Dzierzak, Thomas Gridley, Lluis Espinosa, Anna Bigas
Affiliations
- PMID: 18528438
- PMCID: PMC2486417
- DOI: 10.1038/emboj.2008.113
Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1
Alex Robert-Moreno et al. EMBO J. 2008.
Abstract
Specific deletion of Notch1 and RBPjkappa in the mouse results in abrogation of definitive haematopoiesis concomitant with the loss of arterial identity at embryonic stage. As prior arterial determination is likely to be required for the generation of embryonic haematopoiesis, it is difficult to establish the specific haematopoietic role of Notch in these mutants. By analysing different Notch-ligand-null embryos, we now show that Jagged1 is not required for the establishment of the arterial fate but it is required for the correct execution of the definitive haematopoietic programme, including expression of GATA2 in the dorsal aorta. Moreover, successful haematopoietic rescue of the Jagged1-null AGM cells was obtained by culturing them with Jagged1-expressing stromal cells or by lentiviral-mediated transduction of the GATA2 gene. Taken together, our results indicate that Jagged1-mediated activation of Notch1 is responsible for regulating GATA2 expression in the AGM, which in turn is essential for definitive haematopoiesis in the mouse.
Figures
Figure 1
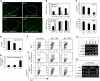
Heterogeneous expression of Notch family members within the aortic haematopoietic clusters. (A) Expression of the indicated genes in the AGM of E10.5 embryos by WISH. Transversal sections of dorsal aorta in a dorsal-to-ventral orientation ( × 400). Details of clustered cells are in the right panels. (B) Immunofluorescence staining of anti-N1ICv (green) overlayed with DAPI (blue) in the AGM region. Details of clusters are in the lower panels. (C) WISH of Notch target genes in clustered cells budding from the dorsal aorta.
Figure 2
Altered haematopoiesis in the AGM of Jagged1 but not Jagged2 mutant embryos. (A) Precisely timed E10.5–11 Ly-6A–GFP, Jag1Δ/Δ/Ly-6A–GFP or Jag2Δ/Δ/Ly-6A–GFP embryos were sectioned and the number of GFP+ cells lining the dorsal aorta was counted. Representative photographs from these embryos are shown. The orientation is dorsal-to-ventral ( × 400). (B) Bars represent the number of GFP+ cells found in 100 μm of AGM aorta from three different Jag1Δ/Δ/Ly-6A–GFP or Jag2Δ/Δ/Ly-6A–GFP embryos compared with their wild-type littermates. (C) Bars represent the number of CFC from Jag1Δ/Δ or Jag2Δ/Δ E10.5 embryos compared with their wild-type littermates. (D) Percent of CD45+ cells obtained in liquid culture of AGM cells from Jag1+/+ and Jag1Δ/Δ. (E) Fold increase in the percent of CD45+ cells when cells are cultured on the OP9 cells. (F) Percent of CD45+ cells obtained from Jag1+/+ and Jag1Δ/Δ AGM cells cultured without stroma or on Jag1+/+ or Jag1Δ/Δ MEF. (G) Expression levels of different Notch ligands in the OP9 cells compared with Jag1+/+ and Jag1Δ/Δ fibroblast and (H) in dissected E10.5 AGM from littermates of different Jag1 genotypes.
Figure 3
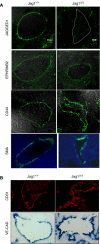
Arterial fate is not affected in the Jagged1 mutant embryos. (A, B) Sections from Jag1+/+ and Jag1Δ/Δ E10.5–11 embryos showing expression of Jag1, EfnB2, CD44, SMA and CD31 by immunofluorescence or VE-cad by WISH. Confocal images for Jag1, EfnB2 and CD44 merged with the Nomarsky image are shown ( × 630). Detection of VE-cadherin (WISH) and immunofluorescence of SMA (merged with DAPI) and CD31 were obtained in Olympus BX60 at × 400.
Figure 4
GATA2 expression is compromised in Jagged1 mutants. (A) WISH for the haematopoietic transcription factors GATA2 and Runx1 in the aortic endothelium of E10.5–11 wild types, Jag1Δ**/**Δ or Jag2Δ/Δ embryos. Somite pair precisely timed embryos were used to compare. The orientation is dorsal-to-ventral ( × 400). (B) Graphs represent the percentage of embryos showing expression of the indicated genes from total analysed embryos. (C) Chromatin IP with the indicated antibodies from six pooled E10.5 dissected AGMs. PCR amplification of the precipitates (left) and relative fold enrichment of the GATA2 promoter as detected by qPCR (right) are shown.
Figure 5
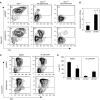
Ectopic GATA2 expression rescues Jagged1 mutant haematopoiesis. (A) Analysis of CD45+ cells by flow cytometry in the GFP+ population obtained from E10.5 AGM cultures transduced with control pHRGFP and pHRhGATA2 lentivirus. Representative Jag1+/+ and two Jag1Δ/Δ with different penetrance are shown. (B) Graph represents the relative fold increase in the percent of CD45+ cells obtained in the GATA2- compared with GFP-transduced Jag1Δ/Δ cultures. (C) Flow cytometry analysis of one representative of three cultures of AGM cells transduced with the indicated lentivirus and incubated with DMSO or DAPT. The percentage of CD45+ cells within the GFP+ population is indicated. (D) Bars represent the average and standard deviation of CFCs obtained from three different cultures.
Similar articles
- Identification of Cdca7 as a novel Notch transcriptional target involved in hematopoietic stem cell emergence.
Guiu J, Bergen DJ, De Pater E, Islam AB, Ayllón V, Gama-Norton L, Ruiz-Herguido C, González J, López-Bigas N, Menendez P, Dzierzak E, Espinosa L, Bigas A. Guiu J, et al. J Exp Med. 2014 Nov 17;211(12):2411-23. doi: 10.1084/jem.20131857. Epub 2014 Nov 10. J Exp Med. 2014. PMID: 25385755 Free PMC article. - Notch signal strength controls cell fate in the haemogenic endothelium.
Gama-Norton L, Ferrando E, Ruiz-Herguido C, Liu Z, Guiu J, Islam AB, Lee SU, Yan M, Guidos CJ, López-Bigas N, Maeda T, Espinosa L, Kopan R, Bigas A. Gama-Norton L, et al. Nat Commun. 2015 Oct 14;6:8510. doi: 10.1038/ncomms9510. Nat Commun. 2015. PMID: 26465397 Free PMC article. - Jagged2 acts as a Delta-like Notch ligand during early hematopoietic cell fate decisions.
Van de Walle I, De Smet G, Gärtner M, De Smedt M, Waegemans E, Vandekerckhove B, Leclercq G, Plum J, Aster JC, Bernstein ID, Guidos CJ, Kyewski B, Taghon T. Van de Walle I, et al. Blood. 2011 Apr 28;117(17):4449-59. doi: 10.1182/blood-2010-06-290049. Epub 2011 Mar 3. Blood. 2011. PMID: 21372153 Free PMC article. - Functional role of Notch signaling in the developing and postnatal heart.
Nemir M, Pedrazzini T. Nemir M, et al. J Mol Cell Cardiol. 2008 Oct;45(4):495-504. doi: 10.1016/j.yjmcc.2008.02.273. Epub 2008 Mar 10. J Mol Cell Cardiol. 2008. PMID: 18410944 Review. - Ligand-dependent Notch signaling in vascular formation.
Kume T. Kume T. Adv Exp Med Biol. 2012;727:210-22. doi: 10.1007/978-1-4614-0899-4_16. Adv Exp Med Biol. 2012. PMID: 22399350 Review.
Cited by
- The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1.
Neves J, Uchikawa M, Bigas A, Giraldez F. Neves J, et al. PLoS One. 2012;7(1):e30871. doi: 10.1371/journal.pone.0030871. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292066 Free PMC article. - Inflammatory signaling regulates hematopoietic stem and progenitor cell development and homeostasis.
Collins A, Mitchell CA, Passegué E. Collins A, et al. J Exp Med. 2021 Jul 5;218(7):e20201545. doi: 10.1084/jem.20201545. Epub 2021 Jun 15. J Exp Med. 2021. PMID: 34129018 Free PMC article. Review. - Blood stem cell-forming haemogenic endothelium in zebrafish derives from arterial endothelium.
Bonkhofer F, Rispoli R, Pinheiro P, Krecsmarik M, Schneider-Swales J, Tsang IHC, de Bruijn M, Monteiro R, Peterkin T, Patient R. Bonkhofer F, et al. Nat Commun. 2019 Aug 8;10(1):3577. doi: 10.1038/s41467-019-11423-2. Nat Commun. 2019. PMID: 31395869 Free PMC article. - Hes1 expression and CYLD repression are essential events downstream of Notch1 in T-cell leukemia.
D'Altri T, Gonzalez J, Aifantis I, Espinosa L, Bigas A. D'Altri T, et al. Cell Cycle. 2011 Apr 1;10(7):1031-6. doi: 10.4161/cc.10.7.15067. Epub 2011 Apr 1. Cell Cycle. 2011. PMID: 21389783 Free PMC article. - Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis.
Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, Aifantis I, Frenette PS, Kitajewski J, Rafii S, Butler JM. Poulos MG, et al. Cell Rep. 2013 Sep 12;4(5):1022-34. doi: 10.1016/j.celrep.2013.07.048. Epub 2013 Sep 5. Cell Rep. 2013. PMID: 24012753 Free PMC article.
References
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 - PubMed
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F et al. (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials