Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: the relationship between stability and amyloidosis - PubMed (original) (raw)
. 2008 Jul 1;47(26):6969-84.
doi: 10.1021/bi800636q. Epub 2008 Jun 7.
Affiliations
- PMID: 18537267
- PMCID: PMC2667099
- DOI: 10.1021/bi800636q
Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: the relationship between stability and amyloidosis
Amy R Hurshman Babbes et al. Biochemistry. 2008.
Abstract
Urea denaturation studies were carried out as a function of transthyretin (TTR) concentration to quantify the thermodynamically linked quaternary and tertiary structural stability and to improve our understanding of the relationship between mutant folding energetics and amyloid disease phenotype. Urea denaturation of TTR involves at least two equilibria: dissociation of tetramers into folded monomers and monomer unfolding. To deal with the thermodynamic linkage of these equilibria, we analyzed concentration-dependent denaturation data by globally fitting them to an equation that simultaneously accounts for the two-step denaturation process. Using this method, the quaternary and tertiary structural stabilities of well-behaved TTR sequences, wild-type (WT) TTR and the disease-associated variant V122I, were scrutinized. The V122I variant is linked to late onset familial amyloid cardiomyopathy, the most common familial TTR amyloid disease. V122I TTR exhibits a destabilized quaternary structure and a stable tertiary structure relative to those of WT TTR. Three other variants of TTR were also examined, L55P, V30M, and A25T TTR. The L55P mutation is associated with the most aggressive familial TTR amyloid disease. L55P TTR has a complicated denaturation pathway that includes dimers and trimers, so globally fitting its concentration-dependent urea denaturation data yielded error-laden estimates of stability parameters. Nevertheless, it is clear that L55P TTR is substantially less stable than WT TTR, primarily because its tertiary structure is unstable, although its quaternary structure is destabilized as well. V30M is the most common mutation associated with neuropathic forms of TTR amyloid disease. V30M TTR is certainly destabilized relative to WT TTR, but like L55P TTR, it has a complex denaturation pathway that cannot be fit to the aforementioned two-step denaturation model. Literature data suggest that V30M TTR has stable quaternary structure but unstable tertiary structure. The A25T mutant, associated with central nervous system amyloidosis, is highly aggregation-prone and exhibits drastically reduced quaternary and tertiary structural stabilities. The observed differences in stability among the disease-associated TTR variants highlight the complexity and heterogeneity of TTR amyloid disease, an observation that has important implications for the treatment of these maladies.
Figures
Figure 1
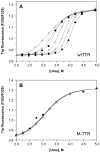
Dependence of urea denaturation of WT TTR (panel A) and M-TTR (panel B) on TTR concentration. Denaturation reactions contained TTR (1.44–144 μM) and were incubated for 96 h at 4 °C in the presence of varying concentrations of urea (0–8 M) prior to fluorescence measurements (see Experimental Procedures for details). The intrinsic tryptophan fluorescence of TTR shifts from a maximum emission wavelength around 335 nm in the native state to 355 nm upon denaturation; hence the ratio of the fluorescence intensity at these two wavelengths (F355/F335) is a measure of the extent of denaturation. TTR concentrations in this representative experiment were 1.44 (❍), 3.6 (□), 7.2 (◆), 14.4 (×), 36 (+), 72 (Δ), and 144μM (λ), and the fits of each denaturation curve to eq 1 are shown as solid lines. The apparent stability of WT TTR monomers to urea denaturation increases with increasing TTR concentration (from Cm,unfold = 3.1 to 4.0 M urea); in contrast the concentration of M-TTR has no effect on its apparent stability (Cm,unfold = 3.1 M urea at all M-TTR concentrations examined).
Figure 2
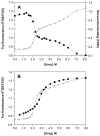
Urea denaturation of WT TTR. The denaturation of WT TTR (36 μM) was monitored both by intrinsic tryptophan fluorescence (❍, left y-axis) to assess changes in the tertiary structure, and by resveratrol binding (■, right y-axis) to assess tetramer dissociation. Samples were incubated for 96 h at 25 °C in the presence of varying concentrations of urea (0–8 M) prior to fluorescence measurements. Each data set was fit to a two-state denaturation model (eq 1 for tryptophan fluorescence data; eq 2 for resveratrol binding data) to give apparent Cm values of 3.8 and 3.7 M urea for tetramer dissociation and monomer unfolding, respectively.
Figure 3
Chemical cross-linking of urea-denatured WT TTR. The same WT TTR samples (36μM) analyzed by fluorescence in Figure 2 were also cross-linked with glutaraldehyde and analyzed by SDS–PAGE with Coomassie Blue staining (see Experimental Procedures for details). Molecular weights of protein standards (Lane 2) are shown on the _y_-axis, and the urea concentration for each of the TTR samples is shown on the _x_-axis. The bands ~55 kDa correspond to TTR tetramers; these disappear as the concentration of urea is increased, with concomitant appearance of a diffuse monomer band ~14 kDa. The bands observed ~42 and 28 kDa probably correspond to trimeric and dimeric TTR, respectively. The intensity of each band was quantified by densitometry (not shown), and the Cm,diss determined by this method was 3.7 or 3.8 M urea, depending on whether the %tetramer or %monomer data were used, in good agreement with the value determined by resveratrol binding (3.8 M; see Figure 2).
Figure 4
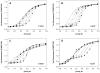
Dependence of urea denaturation of V122I (panel A), L55P (panel B), V30M (panel C), and A25T TTR (panel D) on protein concentration. Denaturation reactions contained TTR (1.44–144μM) and were incubated for 96 h at 4 °C in the presence of varying concentrations of urea (0–8 M) prior to fluorescence measurements (see Experimental Procedures for details). TTR concentrations for each variant were 1.44 (❍), 3.6 (□), 7.2 (◆), 14.4 (×), 36 (+), 72 (Δ), and 144μM (λ). The denaturation curves of V122I (panel A) and L55P (panel B) resemble those observed for WT TTR (see Figure 1A), with the steepness of the transition slope increasing and the curves shifting progressively further to the right with increasing TTR concentration. In contrast, for the remaining variants, V30M (panel C) and A25T (panel D), two transitions are observed in each curve, and the data cannot be fit to a two-state denaturation model (solid lines shown in these panels represent smoothing curves, rather than fits of the data to eq 1). Nevertheless, for all of the variants, the apparent stability of TTR to urea denaturation increases with increasing TTR concentration (Cm increases from 3.3 to 3.8 M urea for V122I, from 1.9 to 2.8 M urea for L55P, from 1.5 to 2.5 M urea for V30M, and from 1.7 to 3.1 M urea for the 2nd transition of A25T; note that the panels have different _x_-axis scales.)
Figure 5
Chemical cross-linking of urea-denatured V122I (panel A), L55P (panel B), V30M (panel C), and A25T (panel D). TTR samples (36 μM for panels A–C and 144 μM for panel D) were cross-linked with glutaraldehyde and analyzed by SDS–PAGE with Coomassie Blue staining (see Experimental Procedures for details). Molecular weights of protein standards are shown on the _y_-axis, and the urea concentration for each of the TTR samples is shown on the _x_-axis. The bands ~55 kDa correspond to TTR tetramers; these disappear as the concentration of urea is increased, with concomitant appearance of a diffuse monomer band ~14 kDa. The denaturation of V122I (panel A) and V30M (panel C) resemble the data obtained for WT TTR (see Figure 3), in that for these 3 variants, the main species observed are the native tetramer and the denatured monomer (although some trimer and dimer are observed for V30M TTR). In contrast, when L55P is denatured (panel B), a significant fraction of dimeric TTR species (seen as bands ~28 and ~23 kDa) accumulate at intermediate urea concentrations. For the A25T variant (panel D), aggregation is observed rather than denaturation at low concentrations of urea. The aggregates formed are large, do not enter the gel, and accumulate maximally around 2.0 M urea; higher concentrations of urea denature the aggregates into monomers.
Figure 6
Urea denaturation of V30M. (A) The denaturation of V30M (36 μM) was monitored both by intrinsic tryptophan fluorescence (❍, left y-axis) to assess changes in the tertiary structure, and by resveratrol binding (■, right y-axis) to assess tetramer dissociation. Samples were incubated for 96 h at 25 °C in the presence of varying concentrations of urea (0–8 M) prior to fluorescence measurements. Using either method, two transitions can be clearly seen in the denaturation curves, and the data cannot be fit to a two-state denaturation model. (B) Curves for the denaturation (❍) and renaturation (■) of V30M (36 μM), assessed by intrinsic tryptophan fluorescence, are compared. For the denaturation experiment, samples were incubated for 96 h at 4 °C in the presence of varying concentrations of urea (0–8 M); note that the lower temperature used here minimizes but does not eliminate the 2nd transition (compare with panel A). For the renaturation experiment, V30M (520 μM) was completely denatured by incubating the sample for 96 h at 4 °C in 8 M urea. The denatured V30M was subsequently diluted into renaturation reactions containing varying concentrations of urea (0.55–8 M), and the samples were incubated for 24 h at 25 °C prior to fluorescence measurements. The 2nd transition observed in the denaturation curve is absent in the renaturation curve; however, V30M cannot be completely refolded under these conditions, and furthermore the two curves do not overlap in the transition region. Solid lines in both panels represent smoothing curves of the data.
Scheme 1
Thermodynamic stability of tetrameric TTR_a_ a Denaturation of tetrameric TTR by urea involves at least two equilibria, as shown–dissociation of the tetramer into its component monomers, which are still natively folded, and subsequent unfolding of the monomers. These equilibria are defined by the equilibrium constants, Kdiss and Kunfold, which can in principle be determined independently from resveratrol binding and intrinsic Trp fluorescence, respectively. The data presented herein, however, show that the equilibria for dissociation and unfolding are thermodynamically linked, meaning that there is a negligible concentration of folded monomer under all the conditions examined. When the equilibria are linked, the apparent value of Kunfold is dependent on TTR concentration, and the concentration-dependence of Kunfold is a measure of the magnitude of Kdiss. Multiple urea denaturation curves at varying TTR concentration can hence be analyzed simultaneously to determine thermodynamic properties of both the tetramers (Kdiss) and the monomers (Kunfold) of wild-type and variant TTRs. Attempts to unlink the equilibria by decreasing the concentration of TTR were unsuccessful, due to limitations in the sensitivity of the measurement techniques.
Similar articles
- The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug.
Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. Johnson SM, et al. J Mol Biol. 2012 Aug 10;421(2-3):185-203. doi: 10.1016/j.jmb.2011.12.060. Epub 2012 Jan 5. J Mol Biol. 2012. PMID: 22244854 Free PMC article. Review. - Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity.
Hammarström P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Hammarström P, et al. Proc Natl Acad Sci U S A. 2002 Dec 10;99 Suppl 4(Suppl 4):16427-32. doi: 10.1073/pnas.202495199. Epub 2002 Sep 25. Proc Natl Acad Sci U S A. 2002. PMID: 12351683 Free PMC article. - Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid.
Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, Miroy GJ, Peterson SA. Kelly JW, et al. Adv Protein Chem. 1997;50:161-81. doi: 10.1016/s0065-3233(08)60321-6. Adv Protein Chem. 1997. PMID: 9338081 Review.
Cited by
- The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug.
Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. Johnson SM, et al. J Mol Biol. 2012 Aug 10;421(2-3):185-203. doi: 10.1016/j.jmb.2011.12.060. Epub 2012 Jan 5. J Mol Biol. 2012. PMID: 22244854 Free PMC article. Review. - The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure.
Boyer DR, Li B, Sun C, Fan W, Zhou K, Hughes MP, Sawaya MR, Jiang L, Eisenberg DS. Boyer DR, et al. Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3592-3602. doi: 10.1073/pnas.1917914117. Epub 2020 Feb 3. Proc Natl Acad Sci U S A. 2020. PMID: 32015135 Free PMC article. - Amyloid seeding of transthyretin by ex vivo cardiac fibrils and its inhibition.
Saelices L, Chung K, Lee JH, Cohn W, Whitelegge JP, Benson MD, Eisenberg DS. Saelices L, et al. Proc Natl Acad Sci U S A. 2018 Jul 17;115(29):E6741-E6750. doi: 10.1073/pnas.1805131115. Epub 2018 Jun 28. Proc Natl Acad Sci U S A. 2018. PMID: 29954863 Free PMC article. - The proteostasis boundary in misfolding diseases of membrane traffic.
Hutt DM, Powers ET, Balch WE. Hutt DM, et al. FEBS Lett. 2009 Aug 20;583(16):2639-46. doi: 10.1016/j.febslet.2009.07.014. FEBS Lett. 2009. PMID: 19708088 Free PMC article. Review. - Identification of a novel transthyretin mutation D39Y in a cardiac amyloidosis patient and its biochemical characterizations.
Ma Q, Wang M, Huang Y, Nie Y, Zhang X, Yang DD, Wang Z, Ding S, Qian N, Liu Y, Pan X. Ma Q, et al. Front Cardiovasc Med. 2023 Jan 26;10:1091183. doi: 10.3389/fcvm.2023.1091183. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36776255 Free PMC article.
References
- Kelly JW. Alternative conformations of amyloidogenic proteins govern their behavior. Curr Opin Struct Biol. 1996;6:11–17. - PubMed
- Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–241. - PubMed
- Buxbaum JN, Tagoe CE. The genetics of the amyloidoses. Annu Rev Med. 2000;51:543–569. - PubMed
- Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. - PubMed
- Lai Z, Colon W, Kelly JW. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM067348-01/GM/NIGMS NIH HHS/United States
- R01 DK046335/DK/NIDDK NIH HHS/United States
- R37 DK046335/DK/NIDDK NIH HHS/United States
- F32 GM067348/GM/NIGMS NIH HHS/United States
- DK46335/DK/NIDDK NIH HHS/United States
- T32-AG00080/AG/NIA NIH HHS/United States
- T32 AG000080/AG/NIA NIH HHS/United States
- T32 AG000080-20/AG/NIA NIH HHS/United States
- F32-GM067348/GM/NIGMS NIH HHS/United States
- R01 DK046335-13/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous