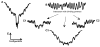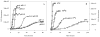Amyloidogenesis of natively unfolded proteins - PubMed (original) (raw)
Review
Amyloidogenesis of natively unfolded proteins
Vladimir N Uversky. Curr Alzheimer Res. 2008 Jun.
Abstract
Aggregation and subsequent development of protein deposition diseases originate from conformational changes in corresponding amyloidogenic proteins. The accumulated data support the model where protein fibrillogenesis proceeds via the formation of a relatively unfolded amyloidogenic conformation, which shares many structural properties with the pre-molten globule state, a partially folded intermediate first found during the equilibrium and kinetic (un)folding studies of several globular proteins and later described as one of the structural forms of natively unfolded proteins. The flexibility of this structural form is essential for the conformational rearrangements driving the formation of the core cross-beta structure of the amyloid fibril. Obviously, molecular mechanisms describing amyloidogenesis of ordered and natively unfolded proteins are different. For ordered protein to fibrillate, its unique and rigid structure has to be destabilized and partially unfolded. On the other hand, fibrillogenesis of a natively unfolded protein involves the formation of partially folded conformation; i.e., partial folding rather than unfolding. In this review recent findings are surveyed to illustrate some unique features of the natively unfolded proteins amyloidogenesis.
Figures
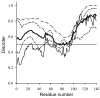
Fig. 1
Intrinsic disorder prediction for human α-synuclein using IUPred (solid line); RONN (dashed line); PONDR VSL2 (dotted line) and PONDR VL3 (dash-dotted line). The results averaged over these for predictions are shown as bold line.
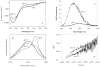
Fig 2
Structural properties and conformational behavior of human α-synuclein. A. Far-UV CD spectra measured under different conditions. B. FTIR spectra measured for natively unfolded, partially folded and fibrilar forms of α-synuclein. C. ANS spectra under different conditions. D. Kratky plot representation of the results of small angle X-ray scattering analysis of α-synuclein at different experimental conditions.
Fig. 3
Conformational behavior human α-synuclein. A. pH-Induced folding of α-synuclein. B. Temperature-induced folding of the nativley unfolded α-synuclein.
Fig. 4
A diagram showing the folding energy landscapes of a typical globular protein (A) [1] and of a typical natively unfolded protein in the absence (B) or presence of different binding partners (C). These landscapes are depicted schematically in one-dimensional cross-section.
Fig. 5
Effect of pH (A) and tempreature (B) on fibrillation of human α-synuclein.
Similar articles
- Conformational constraints for amyloid fibrillation: the importance of being unfolded.
Uversky VN, Fink AL. Uversky VN, et al. Biochim Biophys Acta. 2004 May 6;1698(2):131-53. doi: 10.1016/j.bbapap.2003.12.008. Biochim Biophys Acta. 2004. PMID: 15134647 Review. - Partially folded intermediates as critical precursors of light chain amyloid fibrils and amorphous aggregates.
Khurana R, Gillespie JR, Talapatra A, Minert LJ, Ionescu-Zanetti C, Millett I, Fink AL. Khurana R, et al. Biochemistry. 2001 Mar 27;40(12):3525-35. doi: 10.1021/bi001782b. Biochemistry. 2001. PMID: 11297418 - What does it mean to be natively unfolded?
Uversky VN. Uversky VN. Eur J Biochem. 2002 Jan;269(1):2-12. doi: 10.1046/j.0014-2956.2001.02649.x. Eur J Biochem. 2002. PMID: 11784292 Review. - Predictors of natively unfolded proteins: unanimous consensus score to detect a twilight zone between order and disorder in generic datasets.
Deiana A, Giansanti A. Deiana A, et al. BMC Bioinformatics. 2010 Apr 21;11:198. doi: 10.1186/1471-2105-11-198. BMC Bioinformatics. 2010. PMID: 20409339 Free PMC article. - Natively unfolded human prothymosin alpha adopts partially folded collapsed conformation at acidic pH.
Uversky VN, Gillespie JR, Millett IS, Khodyakova AV, Vasiliev AM, Chernovskaya TV, Vasilenko RN, Kozlovskaya GD, Dolgikh DA, Fink AL, Doniach S, Abramov VM. Uversky VN, et al. Biochemistry. 1999 Nov 9;38(45):15009-16. doi: 10.1021/bi990752+. Biochemistry. 1999. PMID: 10555983
Cited by
- Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro.
Karakani AM, Riazi G, Mahmood Ghaffari S, Ahmadian S, Mokhtari F, Jalili Firuzi M, Zahra Bathaie S. Karakani AM, et al. Iran J Basic Med Sci. 2015 May;18(5):485-92. Iran J Basic Med Sci. 2015. PMID: 26124935 Free PMC article. - Guiding protein aggregation with macromolecular crowding.
Munishkina LA, Ahmad A, Fink AL, Uversky VN. Munishkina LA, et al. Biochemistry. 2008 Aug 26;47(34):8993-9006. doi: 10.1021/bi8008399. Epub 2008 Jul 30. Biochemistry. 2008. PMID: 18665616 Free PMC article. - Chain collapse of an amyloidogenic intrinsically disordered protein.
Jain N, Bhattacharya M, Mukhopadhyay S. Jain N, et al. Biophys J. 2011 Oct 5;101(7):1720-9. doi: 10.1016/j.bpj.2011.08.024. Biophys J. 2011. PMID: 21961598 Free PMC article. - Pulsed Hydrogen-Deuterium Exchange Illuminates the Aggregation Kinetics of α-Synuclein, the Causative Agent for Parkinson's Disease.
Illes-Toth E, Rempel DL, Gross ML. Illes-Toth E, et al. ACS Chem Neurosci. 2018 Jun 20;9(6):1469-1476. doi: 10.1021/acschemneuro.8b00052. Epub 2018 Apr 11. ACS Chem Neurosci. 2018. PMID: 29601177 Free PMC article. - Landscape of Pleiotropic Proteins Causing Human Disease: Structural and System Biology Insights.
Ittisoponpisan S, Alhuzimi E, Sternberg MJ, David A. Ittisoponpisan S, et al. Hum Mutat. 2017 Mar;38(3):289-296. doi: 10.1002/humu.23155. Epub 2017 Jan 11. Hum Mutat. 2017. PMID: 27957775 Free PMC article.
References
- Onuchic JN, Nymeyer H, Garcia AE, Chahine J, Socci ND. The energy landscape theory of protein folding: insights into folding mechanisms and scenarios. Adv Protein Chem. 2000;53:87–152. - PubMed
- Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–106. - PubMed
- Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. - PubMed
- Uversky VN, Talapatra A, Gillespie JR, Fink AL. Protein Deposits As the Molecular Basis of Amyloidosis. II. Localized Amyloidosis and Neurodegenerative Disordres. Med Sci Monitor. 1999;5:1238–1254.