TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane - PubMed (original) (raw)
TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane
Sebastian Brauchi et al. Proc Natl Acad Sci U S A. 2008.
Abstract
TRPM7, of the transient receptor potential (TRP) family, is both an ion channel and a kinase. Previously, we showed that TRPM7 is located in the membranes of acetylcholine (ACh)-secreting synaptic vesicles of sympathetic neurons, forms a molecular complex with proteins of the vesicular fusion machinery, and is critical for stimulated neurotransmitter release. Here, we targeted pHluorin to small synaptic-like vesicles (SSLV) in PC12 cells and demonstrate that it can serve as a single-vesicle plasma membrane fusion reporter. In PC12 cells, as in sympathetic neurons, TRPM7 is located in ACh-secreting SSLVs. TRPM7 knockdown by siRNA, or abolishing channel activity by expression of a dominant negative TRPM7 pore mutant, decreased the frequency of spontaneous and voltage-stimulated SSLV fusion events without affecting large dense core vesicle secretion. We conclude that the conductance of TRPM7 across the vesicle membrane is important in SSLV fusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
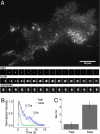
Endogenous TRPM7 in SSLVs in PC12 cells. (A) Sucrose gradient distribution of the PC12 postnuclear supernatant membranes. Western blots show the association of TRPM7, synaptophysin, and VAChT (+/− pHluorin tag) with SSLV fractions. Expressed VAChT-pHluorin was detected with GFP antibody. (B) Immunofluorescent confocal image of a nontransfected PC12 cell labeled by anti-TRPM7 antibody. TRPM7 is labeled in vesicles packing the cell cytoplasm and is excluded from the nucleus. (Scale bar: 5 μm.)
Fig. 2.
Cholinergic vesicle fusion in PC12 cells monitored by VAChT-pHluorin fluorescence. (A) Single-frame TIRF image of PC12 cell expressing VAChT-pHluorin. (Insets) Selected frame sequences from fast, slow, and sustained events. (B) Example of fluorescence intensity decay time courses for transient (fast) and full-fusion (slow) events. Solid lines are the single exponential fit of the measured points. (C) Bar plot summarizes the kinetics of flash fluorescence decay in VAChT-pHluorin-expressing PC12 cells. The events segregate into fast (τ = 0.68 ± 0.28 s) and slow (τ = 3.25 ± 0.42 s) populations. Shown are 54 total events in 20 cells. (Error bars: ± SEM.)
Fig. 3.
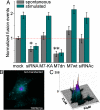
Reduction in TRPM7 channel activity decreases SSLV fusion event frequency. (A) Population analysis of the spontaneous and stimulation-evoked SSLV fusion events with the plasma membrane. For each recorded cell, the total number of fusion events (transient, full, and sustained) in 300 frames was divided by the cell area and normalized to the average accumulated fusion number in vector-transfected cells. TRPM7 knockdown (siRNA; P < 0.05; 118 total recorded fusion events, 9 cells) and dnTRPM7 expression (dnM7; P < 0.001; 132 total recorded fusion events, 12 cells) substantially reduced fusion frequency. In contrast, overexpression of wt TRPM7 (M7-wt) and kinase-dead TRPM7 (M7-KA) did not alter fusion frequency. Fusion frequency was not significantly different in mock-transfected cells compared with nontransfected cells (data not shown). A total of 56 cells were recorded. (Error bars: ± SEM.) (B and C) Example illustrating SSLV fusions inhibition with dnTRPM7. (B) Simultaneous imaging of nontransfected and dnTRPM7-transfected cells. Image is the sum of 300 background-subtracted frames. (C) Three-dimensional projection of intensity (z; arbitrary units) and x, y pixel location of the image in A.
Fig. 4.
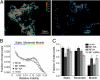
Increased vesicle mobility in kinase-dead TRPM7-transfected PC12 cells. (A) Color-coded mobility images reflect variation of a nonfusing vesicle's position within a 30-s recording (300 frames, see Materials and Methods). (Left) TRPM7-KA transfected cell. (Right) Mock-transfected cell. (B) Distribution histogram showing fraction of pixels with the specified mobility. Relative mobility values are defined in the Materials and Methods. Bars at top define the regions integrated. (C) Population statistics of the integrated relative pixel mobility in TRPM7-transfected PC12 cells. Pixels were separated into three groups (static: 0.01 < δ < 0.09; moderately mobile: 0.091 < δ < 0.17; and highly mobile: 0.171 < δ < 0.253). (Error bars: ± SEM.)
Similar articles
- The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release.
Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. Krapivinsky G, et al. Neuron. 2006 Nov 9;52(3):485-96. doi: 10.1016/j.neuron.2006.09.033. Neuron. 2006. PMID: 17088214 - [TRPM7 ion channel in cholinergic synaptic vesicles].
Mochida S. Mochida S. Tanpakushitsu Kakusan Koso. 2008 Mar;53(4 Suppl):442-7. Tanpakushitsu Kakusan Koso. 2008. PMID: 21089317 Review. Japanese. No abstract available. - TRPM7 is critical for short-term synaptic depression by regulating synaptic vesicle endocytosis.
Jiang ZJ, Li W, Yao LH, Saed B, Rao Y, Grewe BS, McGinley A, Varga K, Alford S, Hu YS, Gong LW. Jiang ZJ, et al. Elife. 2021 Sep 27;10:e66709. doi: 10.7554/eLife.66709. Elife. 2021. PMID: 34569930 Free PMC article. - Protein kinase C-mediated translocation of secretory vesicles to plasma membrane and enhancement of neurotransmitter release from PC12 cells.
Shoji-Kasai Y, Itakura M, Kataoka M, Yamamori S, Takahashi M. Shoji-Kasai Y, et al. Eur J Neurosci. 2002 Apr;15(8):1390-4. doi: 10.1046/j.1460-9568.2002.01972.x. Eur J Neurosci. 2002. PMID: 11994133 - Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes.
Chubanov V, Mederos y Schnitzler M, Wäring J, Plank A, Gudermann T. Chubanov V, et al. Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):334-41. doi: 10.1007/s00210-005-1056-4. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15902429 Review.
Cited by
- Structure of the mammalian TRPM7, a magnesium channel required during embryonic development.
Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, Abiria SA, Krapivinsky G, Zhang J, Clapham DE. Duan J, et al. Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):E8201-E8210. doi: 10.1073/pnas.1810719115. Epub 2018 Aug 14. Proc Natl Acad Sci U S A. 2018. PMID: 30108148 Free PMC article. - A Novel Role of the TRPM4 Ion Channel in Exocytosis.
Stokłosa P, Kappel S, Peinelt C. Stokłosa P, et al. Cells. 2022 May 30;11(11):1793. doi: 10.3390/cells11111793. Cells. 2022. PMID: 35681487 Free PMC article. - The modulation of TRPM7 currents by nafamostat mesilate depends directly upon extracellular concentrations of divalent cations.
Chen X, Numata T, Li M, Mori Y, Orser BA, Jackson MF, Xiong ZG, MacDonald JF. Chen X, et al. Mol Brain. 2010 Dec 1;3:38. doi: 10.1186/1756-6606-3-38. Mol Brain. 2010. PMID: 21122141 Free PMC article. - The spatial pattern of exocytosis and post-exocytic mobility of synaptopHluorin in mouse motor nerve terminals.
Gaffield MA, Tabares L, Betz WJ. Gaffield MA, et al. J Physiol. 2009 Mar 15;587(Pt 6):1187-200. doi: 10.1113/jphysiol.2008.166728. Epub 2009 Jan 19. J Physiol. 2009. PMID: 19153160 Free PMC article. - Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7.
Chubanov V, Mederos y Schnitzler M, Meißner M, Schäfer S, Abstiens K, Hofmann T, Gudermann T. Chubanov V, et al. Br J Pharmacol. 2012 Jun;166(4):1357-76. doi: 10.1111/j.1476-5381.2012.01855.x. Br J Pharmacol. 2012. PMID: 22242975 Free PMC article.
References
- Galli T, Haucke V. Cycling of synaptic vesicles: How far?. How fast! Sci STKE. 2001;2001:RE1. - PubMed
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
- Rahamimoff R, Fernandez JM. Pre- and postfusion regulation of transmitter release. Neuron. 1997;18:17–27. - PubMed
- Stanley EF, Ehrenstein G, Russell JT. Evidence for anion channels in secretory vesicles. Neuroscience. 1988;25:1035–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous