FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage - PubMed (original) (raw)
FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage
Carolyn A Foster et al. Brain Pathol. 2009 Apr.
Abstract
FTY720 (fingolimod) is an oral sphingosine-1 phosphate (S1P) receptor modulator in phase III development for the treatment of multiple sclerosis. To further investigate its mode of action, we analyzed gene expression in the central nervous system (CNS) during experimental autoimmune encephalomyelitis (EAE). FTY720 downregulated inflammatory genes in addition to vascular adhesion molecules. It decreased the matrix metalloproteinase gene MMP-9 and increased its counterregulator--tissue inhibitor of metalloproteinase, TIMP-1--resulting in a proteolytic balance that favors preservation of blood-brain-barrier (BBB) integrity. Furthermore, FTY720 reduced S1P lyase that increases the S1P concentration in the brain, in line with a marked reversal of neurological deficits and raising the possibility for enhanced triggering of S1P receptors on resident brain cells. This is accompanied by an increase in S1P(1) and S1P(5) in contrast with the attenuation of S1P(3) and S1P(4). Late-stage rescue therapy with FTY720, even up to 1 month after EAE onset, reversed BBB leakiness and reduced demyelination, along with normalization of neurologic function. Our results indicate rapid blockade of ongoing disease processes by FTY720, and structural restoration of the CNS parenchyma, which is likely caused by the inhibition of autoimmune T cell infiltration and direct modulation of microvascular and/or glial cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1
FTY720 efficacy in DA rat model of chronic‐protracted EAE, actively induced by syngeneic neuroantigens. Data are shown as mean clinical score ± SEM. All animals developed full hind limb paralysis by day 12 and continued to show strong neurologic deficits by the initiation of treatment on days 12 and 40. In contrast with vehicle (▪), oral FTY720 at 0.3 mg/kg completely prevented onset of EAE symptoms upon prophylactic treatment (□). FTY720 0.3 mg/kg therapeutic dosing (○) from days 12 to 28 rapidly and fully curtailed further development of EAE symptoms. Even more importantly, FTY720 rescue therapy at 0.3 mg/kg (◊) from days 40 to 53 significantly reversed neurological impairment to a deficit in tail tonus. On days 11 and 29 (▴ along x‐axis), rats were sacrificed for real‐time PCR (n = 6 per group, including naïve rats). On days 40 and 54 (▵ along x‐axis), animals were sacrificed for neuropathology (n = 6 per group) as shown in 8, 9. Level of significance between the vehicle and FTY720 groups was determined by one‐way ANOVA of area‐under‐the‐curve values from days 0–11, 12–29 and 40–54 for the prophylactic, therapeutic and rescue therapy arms, respectively, followed by a post‐hoc Tukey test. ***P ≤ 0.001. Abbreviations: FTY720 = fingolimod; DA = Dark Agouti; EAE = experimental autoimmune encephalomyelitis; SEM = standard error of mean; PCR = polymerase chain reaction; ANOVA = analysis of variance.
Figure 2
Real‐time PCR expression profile of representative inflammatory and leukocyte‐associated genes in EAE‐diseased spinal cord of DA rats, compared with FTY720 treatment. The two time points represent tissue sampling on days 11 and 29 following preventive and therapeutic treatment respectively, as depicted in Figure 1. Data represent pooled samples from cervical, thoracic and lumbar spinal cord segments (n = 3 rats/group, nine samples each), indicated as mean expression ± SEM relative to the naïve group. Panels A and B show five to 10‐fold differences between vehicle ( ) and FTY70 (▪) on days 11 and 29. Panels C and D demonstrate differences between the two groups that are >10‐fold at one or both time points. Expression levels with <fivefold differences (Appendix 2), even if significant for one or both days, are not shown. Level of significance between the vehicle and FTY720 groups was determined by a student _t_‐test for each gene. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: PCR = polymerase chain reaction; EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; FTY720 = fingolimod; SEM = standard error of mean.
) and FTY70 (▪) on days 11 and 29. Panels C and D demonstrate differences between the two groups that are >10‐fold at one or both time points. Expression levels with <fivefold differences (Appendix 2), even if significant for one or both days, are not shown. Level of significance between the vehicle and FTY720 groups was determined by a student _t_‐test for each gene. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: PCR = polymerase chain reaction; EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; FTY720 = fingolimod; SEM = standard error of mean.
Figure 3
Expression of extravasation and endothelial barrier complex genes by real‐time PCR in EAE‐diseased spinal cord from DA rats on days 11 and 29 postimmunization, compared with FTY720 treatment. Data represent pooled samples from cervical, thoracic and lumbar spinal cord segments (n = 3 rats/group, nine samples each), depicted as mean expression ± SEM. Gene induction levels that we referred to in the Results were calculated as the fold‐increase relative to naïve (not shown); these sometimes differed to the absolute expression values shown here. For example, spinal cord expression ± SEM in the naïve animals (n = 6 rats) ranged from 0.93 ± 0.08 (ICAM‐1), 1.53 ± 0.46 (MMP‐9), 1.67 ± 0.15 (P‐selectin), 1.52 ± 0.14 (TIMP‐1) to 0.77 ± 0.03 (VCAM‐1). Significant differences between gene expression in the vehicle ( ) versus FTY70 (▪) groups are indicated, as determined by student t‐test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: PCR = polymerase chain reaction; EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; FTY720 = fingolimod; SEM = standard error of mean; ns = not significant.
) versus FTY70 (▪) groups are indicated, as determined by student t‐test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: PCR = polymerase chain reaction; EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; FTY720 = fingolimod; SEM = standard error of mean; ns = not significant.
Figure 4
Time course of gene expression ± SEM in the brain stem and cerebellum. In most cases, vehicle‐treated animals showed a significant and reversed temporal difference in genes expressed in the brain stem and cerebellum at day 11 compared with day 29, indicated by brackets on the top of each panel. There was no significant variation in the six genes examined between day 11 and 29 in the FTY720 treatment group or compared with the naïve animals, except for a difference on day 11 in the brain stem (P = 0.04) for MCP‐1 between naïve animals. Expression ± SEM in the naïve animals (n = 6 rats) included the following for brain stem and cerebellum respectively: 0.75 ± 0.06 and 1.19 ± 0.08 (BL34), 0.39 ± 0.13 and 0.66 ± 0.14 (IL‐1β), 0.82 ± 0.05 and 1.20 ± 0.06 (ICAM‐1), 0.66 ± 0.09 and 0.80 ± 0.05 (MCP‐1), 0.31 ± 0.15 and 0.95 ± 0.14 (MMP‐9), 0.89 ± 0.04 and 1.19 ± 0.04 (TIMP‐1). Moreover, gene induction levels that we referred to in the Results were calculated as the fold‐increase relative to naïve (not shown); these sometimes differed to the absolute expression values shown here. Significant differences in gene expression between the vehicle and FTY720 groups, as determined by student _t_‐test, are shown at days 11 and 29 after prophylactic and therapeutic treatment, respectively. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: d = day; ns = not significant.
Figure 5
Expression of genes encoding myelin proteins. Data are depicted as mean expression ± SEM in the spinal cord and brain stem for (A) myelin basic protein (MBP), (B) myelin oligodendrocyte glycoprotein (MOG), and (C) proteolipid protein (PLP); the latter also included assessment in the cerebellum. There was no difference between FTY720 and naïve animals except for MOG on day 11 in the brain stem. Compared with the vehicle group, FTY720‐treated animals displayed a significant increase in all 3 myelin proteins in the spinal cord samples following prophylactic and therapeutic dosing (A–C). There was, in addition, a marked difference in PLP expression between vehicle and FTY720 in the brain stem on day 11. Otherwise, in the cerebellar and remaining brain stem samples expression values for naïve, FTY720 and vehicle all converged at a similar level, especially by day 29. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: SEM = standard error of mean; FTY720 = fingolimod; EAE = experimental autoimmune encephalomyelitis; NS = not significant.
Figure 6
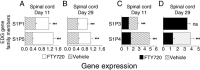
Expression of EDG family genes that are modulated by FTY720, shown as mean ± SEM. (A–B) S1P1 and S1P5 expression in the spinal cord is downregulated in the diseased animals ( ; n = 6) and significantly increased by FTY720 0.3 mg/kg treatment (□; n = 6). (C–D) The reciprocal situation occurs for S1P3 and S1P4 expression in the spinal cord, as these receptors are upregulated in the vehicle controls (
; n = 6) and significantly increased by FTY720 0.3 mg/kg treatment (□; n = 6). (C–D) The reciprocal situation occurs for S1P3 and S1P4 expression in the spinal cord, as these receptors are upregulated in the vehicle controls ( ; n = 6) and reduced by FTY720 treatment (▪; n = 6); an exception is no difference in S1P3 on day 29. Spinal cord expression ± SEM of the S1P receptors in naïve animals (n = 6 rats) ranged from 1.16 ± 0.05 (S1P1), 1.07 ± 0.06 (S1P3), 0.60 ± 0.051 (S1P4) to 1.20 ± 0.06 (S1P5). ***P ≤ 0.001. Abbreviations: FTY720 = fingolimod; DA = Dark Agouti; EAE = experimental autoimmune encephalomyelitis; S1P = sphingosine‐1 phosphate; SEM = standard error of mean; ns = not significant.
; n = 6) and reduced by FTY720 treatment (▪; n = 6); an exception is no difference in S1P3 on day 29. Spinal cord expression ± SEM of the S1P receptors in naïve animals (n = 6 rats) ranged from 1.16 ± 0.05 (S1P1), 1.07 ± 0.06 (S1P3), 0.60 ± 0.051 (S1P4) to 1.20 ± 0.06 (S1P5). ***P ≤ 0.001. Abbreviations: FTY720 = fingolimod; DA = Dark Agouti; EAE = experimental autoimmune encephalomyelitis; S1P = sphingosine‐1 phosphate; SEM = standard error of mean; ns = not significant.
Figure 7
Western blot analysis of cPLA2 protein levels in the cerebellum and spinal cord from an EAE study in DA rats, immunized with syngeneic CNS antigens. (A) Nitrocellulose membrane with cerebellum samples, probed with anti‐cPLA2 antibodies or antibodies to actin as a control for total protein input. The samples were pooled from two to three rats in each experimental group and include CFA‐injected adjuvant control (lane a), EAE‐immunized/vehicle treated (lanes b, e, f), EAE‐immunized/treated with 0.05 mg/kg FTY720 (lane c), or with 0.3 mg/kg FTY720 (lane d); RBL‐2H3 cell lysate was used as a positive control (lane g). FTY720 and vehicle treatment was performed in a therapeutic setting from days 12 to 28 postimmunization. (B) Scans of cPLA2 Western blotting signals ± SEM for cerebellum and spinal cord samples. The band intensity was adjusted to 100% for the negative (adjuvant) control. Diseased animals showed a clear trend toward elevated cPLA2, in contrast with a reduction to background levels under FTY720 (0.3 mg/kg) treatment. Differences between the vehicle and FTY720 were analyzed by a one‐way ANOVA, followed by a post‐hoc Tukey test. Abbreviations: EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; CNS = central nervous system; CFA = complete Freund's adjuvant; FTY720 = fingolimod; SEM = standard error of mean; PCR = polymerase chain reaction; ANOVA = analysis of variance.
Figure 8
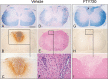
Representative EAE spinal cord histopathology in DA rats treated on days 40–53 with vehicle (A–F) or 0.3 mg/kg FTY720 (G–I). Luxol fast blue staining of cervical and thoracic cross‐sections of the spinal cord shows large areas of confluent demyelination in control rats (A,D). Following FTY720 therapy, confluent demyelination is restricted to the dorsal column of the cervical cord (G, arrow); traces of perivascular demyelination are found in the thoracic cord (data not shown). Serial cross‐sections from spinal cords A,D (vehicle) and G (FTY720) also were stained with H&E and for antirat Ig. In the vehicle controls, active demyelination is associated with extensive Ig precipitation (B,C), reflecting BBB damage; perivascular infiltrates are present within the lesion, as well as in nondemyelinated areas (F). In contrast, FTY720‐treated animals show inactive demyelination with no Ig deposition (H). H&E reveals only sparse infiltration of the lesion (I), mainly containing ED1+ macrophages (data not shown); no perivascular inflammatory infiltrates are present within the lesion. Scale bar, depicted in A for all = 500 µm (A,B,D,E,G,H), 260 µm (C,I), and 30 µm (F). Abbreviations: EAE = experimental autoimmune encephalomyelitis; DA = Dark Agouti; FTY720 = fingolimod; BBB = blood‐brain‐barrier; H&E = hematoxylin and eosin; Ig = immunoglobulin.
Figure 9
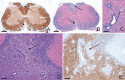
Neuropathology in EAE‐diseased DA rats (n = 4) on day 40, demonstrating representative images of brain and spinal cord damage after immunization with syngeneic CNS antigens and prior to initiation of FTY720 rescue therapy. Sequential cross‐sections of spinal cord show overlying areas of focal demyelination (A, anti‐MBP) and inflammation (B, H&E), as highlighted by the arrows; (C) high‐power H&E depicts typical perivascular inflammation. Sections of the cerebellum illustrate areas of extensive inflammation (D, H&E) which coincides with demyelination (E, anti‐MBP), exemplified by the arrows. Scale bars: A, B = 330 µm; C = 60 µm; D,E = 200 µm. Abbreviations: DA = Dark Agouti; EAE = experimental autoimmune encephalomyelitis; CNS = central nervous system; FTY720 = fingolimod; anti‐MBP = myelin basic protein; H&E = hematoxylin and eosin.
Similar articles
- FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis.
Balatoni B, Storch MK, Swoboda EM, Schönborn V, Koziel A, Lambrou GN, Hiestand PC, Weissert R, Foster CA. Balatoni B, et al. Brain Res Bull. 2007 Oct 19;74(5):307-16. doi: 10.1016/j.brainresbull.2007.06.023. Epub 2007 Jul 30. Brain Res Bull. 2007. PMID: 17845905 - FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation.
Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. Choi JW, et al. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):751-6. doi: 10.1073/pnas.1014154108. Epub 2010 Dec 21. Proc Natl Acad Sci U S A. 2011. PMID: 21177428 Free PMC article. - FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system.
Brinkmann V. Brinkmann V. Br J Pharmacol. 2009 Nov;158(5):1173-82. doi: 10.1111/j.1476-5381.2009.00451.x. Epub 2009 Oct 8. Br J Pharmacol. 2009. PMID: 19814729 Free PMC article. Review. - Central nervous system-directed effects of FTY720 (fingolimod).
Miron VE, Schubart A, Antel JP. Miron VE, et al. J Neurol Sci. 2008 Nov 15;274(1-2):13-7. doi: 10.1016/j.jns.2008.06.031. Epub 2008 Aug 3. J Neurol Sci. 2008. PMID: 18678377 Review. - Fingolimod (FTY720), sphingosine 1-phosphate receptor modulator, shows superior efficacy as compared with interferon-β in mouse experimental autoimmune encephalomyelitis.
Chiba K, Kataoka H, Seki N, Shimano K, Koyama M, Fukunari A, Sugahara K, Sugita T. Chiba K, et al. Int Immunopharmacol. 2011 Mar;11(3):366-72. doi: 10.1016/j.intimp.2010.10.005. Epub 2010 Oct 16. Int Immunopharmacol. 2011. PMID: 20955831
Cited by
- Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier.
van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, van der Pol SM, Geerts D, van Horssen J, van der Valk P, van der Kam E, Ronken E, Reijerkerk A, de Vries HE. van Doorn R, et al. J Neuroinflammation. 2012 Jun 20;9:133. doi: 10.1186/1742-2094-9-133. J Neuroinflammation. 2012. PMID: 22715976 Free PMC article. - Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis.
Martín R, Hernández M, Córdova C, Nieto ML. Martín R, et al. Br J Pharmacol. 2012 Jul;166(5):1708-23. doi: 10.1111/j.1476-5381.2012.01869.x. Br J Pharmacol. 2012. PMID: 22260389 Free PMC article. - The alliance of sphingosine-1-phosphate and its receptors in immunity.
Rivera J, Proia RL, Olivera A. Rivera J, et al. Nat Rev Immunol. 2008 Oct;8(10):753-63. doi: 10.1038/nri2400. Nat Rev Immunol. 2008. PMID: 18787560 Free PMC article. Review. - Lyase to live by: sphingosine phosphate lyase as a therapeutic target.
Kumar A, Saba JD. Kumar A, et al. Expert Opin Ther Targets. 2009 Aug;13(8):1013-25. doi: 10.1517/14728220903039722. Expert Opin Ther Targets. 2009. PMID: 19534571 Free PMC article. Review. - Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis.
Rau CR, Hein K, Sättler MB, Kretzschmar B, Hillgruber C, McRae BL, Diem R, Bähr M. Rau CR, et al. Am J Pathol. 2011 Apr;178(4):1770-81. doi: 10.1016/j.ajpath.2011.01.003. Epub 2011 Feb 26. Am J Pathol. 2011. PMID: 21406175 Free PMC article.
References
- Balatoni B, Storch MK, Swoboda EM, Schönborn V, Koziel A, Lambrou GN et al (2007) FTY720 sustains and restores neuronal function in MOG‐induced experimental autoimmune encephalomyelitis. Brain Res Bull 74:307–316. - PubMed
- Bandhuvula P, Tam YY, Oskouian B, Saba JD (2005) The immune modulator FTY720 inhibits sphingosine‐1‐phosphate lyase activity. J Biol Chem 280:33697–33700. - PubMed
- Barral DC, Brenner MB (2007) CD1 antigen presentation: how it works. Nat Rev Immunol 7:929–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous