Phosphorylation and kinetics of mammalian cytochrome c oxidase - PubMed (original) (raw)
Phosphorylation and kinetics of mammalian cytochrome c oxidase
Stefan Helling et al. Mol Cell Proteomics. 2008 Sep.
Abstract
The influence of protein phosphorylation on the kinetics of cytochrome c oxidase was investigated by applying Western blotting, mass spectrometry, and kinetic measurements with an oxygen electrode. The isolated enzyme from bovine heart exhibited serine, threonine, and/or tyrosine phosphorylation in various subunits, except subunit I, by using phosphoamino acid-specific antibodies. The kinetics revealed slight inhibition of oxygen uptake in the presence of ATP, as compared with the presence of ADP. Mass spectrometry identified the phosphorylation of Ser-34 at subunit IV and Ser-4 and Thr-35 at subunit Va. Incubation of the isolated enzyme with protein kinase A, cAMP, and ATP resulted in serine and threonine phosphorylation of subunit I, which was correlated with sigmoidal inhibition kinetics in the presence of ATP. This allosteric ATP-inhibition of cytochrome c oxidase was also found in rat heart mitochondria, which had been rapidly prepared in the presence of protein phosphatase inhibitors. The isolated rat heart enzyme, prepared from the mitochondria by blue native gel electrophoresis, showed serine, threonine, and tyrosine phosphorylation of subunit I. It is concluded that the allosteric ATP-inhibition of cytochrome c oxidase, previously suggested to keep the mitochondrial membrane potential and thus the reactive oxygen species production in cells at low levels, occurs in living cells and is based on phosphorylation of cytochrome c oxidase subunit I.
Figures
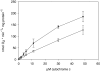
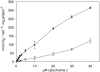
Fig. 1.
Kinetics of CcO in isolated bovine heart mitochondria. Mitochondria were isolated in the presence of 25 m
m
NaF, 1 m
m
vanadate, and 2 m
m
EGTA. Oxygen consumption was measured at increasing concentrations of cytochrome c in the presence of 5 m
m
ADP (closed squares) and 5 m
m
ATP and an ATP regenerating system (open squares). The average of three determinations is shown.
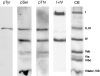
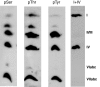
Fig. 2.
Western blots and Coomassie Blue staining (CB) of CcO from bovine heart, purified by standard procedures from mitochondria isolated in the presence of 25 mm NaF, 1 mm sodium vanadate, and 2 mm EGTA. Mitochondria were isolated immediately after obtaining the heart from the slaugtherhouse. Antibodies against phosphotyrosine (pTyr), phosphoserine (pSer), phosphothreonine (pThr), and CcO subunits I and IV were applied, as indicated.
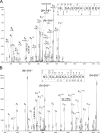
Fig. 3.
NanoLC-ESI-MS/MS analysis of the CcO subunit IV. The MS/MS spectra (A, CID and B, ETD) was unambiguously assigned to the peptide NLSAPSQKALKEKE. Localization of Ser-34 as site of phosphorylation was possible after fragmentation of the triply charged precursor ion m/z 552.2. A, the CID-MS/MS spectrum contains a CID-typical neutral loss of phosphoric acid from the phosphoserine of the precursor ion. The neutral loss ions y10 and y12 as well as the b3 ion indicates a phosphorylated Ser-34. B, both, z9 + 1H and z10 + 1H (both single charged with additional hydrogen) in the ETD-MS/MS experiment additionally confirm the findings of CID fragmentation.
Fig. 4.
NanoLC-ESI-MS/MS analysis of CcO subunit Va. Phosphoserine-4 in CcO subunit Va was unequivocally localized by a combination of CID (A) and ETD (B) of the triply charged precursor ion SHGPSHETDEEFDAR m/z 566.3. The spectrum shows CID-typical neutral loss of phosphoric acid from the precursor ion. The neutral loss containing ions b6+2 and y12+2 indicate the phosphorylation of Ser-4. Furthermore, the occurrence of b2 and y8 ion excludes phosphorylation at Ser-1 and Thr-7. The ETD-MS/MS spectrum additionally confirms a clear localization of Ser-4 phosphorylation by the presence of N-terminal c3 and c4 ions as well as the C-terminal z10 and z11 ions. Additionally, phosphorylated Thr-35 was localized in the triply charged precursor ion GMNPTLVGYDLVPEPK m/z 571.6 using a combination of CID (C) and ETD (D). The CID-MS/MS spectrum shows two abundant ions, the y4 ion and the corresponding N-terminal b11+2 ion, pointing to breakage at the labile peptide bondage in front of Pro-43. The tyrosine phosphorylation (Tyr-39) was excluded by the occurrence of b7, b8, y7, and y9 ions. Furthermore, ETD fragmentation resulted in an easily interpretable spectrum giving a good explanation of the amino acid sequence including the c7 and c8 ions and the corresponding z7 and z8 ions. On the basis of the fragmentation pattern Tyr-39 could be excluded to be phosphorylated, and the c4, z13, and y14 ions explicitly confirm a phosphorylation of Thr-35.
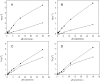
Fig. 5.
Incubation of isolated CcO from bovine heart with PKA, cAMP, and ATP induces the allosteric ATP-inhibition. CcO was incubated for 40 min at 30 °C with (A and B) or without PKA (C and D), as described under “Experimental Procedures.” Before incubation part of the enzyme was dialyzed overnight with cardiolipin (A and C). Oxygen consumption was measured at increasing concentrations of cytochrome c in the presence of 5 m
m
ADP (closed squares) and 5 m
m
ATP and an ATP regenerating system (open squares). The turnover number (TN) is presented as μmol cytochrome c × μmol heme aa3−1 × s−1.
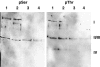
Fig. 6.
Treatment of isolated CcO from bovine heart with PKA, cAMP, and ATP leads to serine (pSer) and threonine phosphorylation (pThr) of subunit I. CcO was incubated for 40 min at 30 °C with (1, 2) or without PKA (3, 4), as described under “Experimental Procedures.” Part of the enzyme was dialyzed overnight with cardiolipin (1, 3).
Fig. 7.
Kinetics of CcO in rat heart mitochondria, isolated immediately after death of the animal in the presence of 25 mm NaF, 5 mm vanadate, 10 nm okadaic acid, 2 mm EGTA, and 0.2% bovine serum albumin. Oxygen consumption was measured at increasing concentrations of cytochrome c in the presence of 5 m
m
ADP (closed squares) and 5 m
m
ATP and an ATP regenerating system (open squares). The average of three determinations is shown.
Fig. 8.
Western blot of rat heart CcO, isolated by blue native gel electrophoresis, with antibodies against phosphoserine (pSer), phosphothreonine (pThr), phosphotyrosine (pTyr), and CcO subunits I and IV. The immunoreactive spot between subunits I and II/III does not correspond to a CcO subunit.
Fig. 9.
Crystal structure of dimeric bovine heart CcO (12) with indication of known phosphorylation sites. Polypeptides above subunit I, presented in green and mainly located within the lipid membrane space, are located in the intermembrane space, those below subunit I in the matrix space. The phosphorylation sites are visualized in both monomers by bold atoms of the corresponding amino acids: Tyr-304 of subunit I (36) in green; Ser-115 + Ser-116 of subunit I; Ser-52 (in rabbit = Thr-52) in subunit IV; and Ser-40 in subunit Vb (39) in blue; Ser-34 in subunit IV, and Ser-4 (indicated by the Cα atom of His-5 because Ser-4 is not in the crystal structure) and Thr-35 in subunit Va (this paper) in red. Subunit II is shown in dark blue. The transmembrane subunit IV and subunit VIa, located at the interface between the two monomers, are presented in red, subunit Va in light blue, and subunit Vb (and VIII) in lila. The two heme a groups are in black, with iron and copper atoms in brown. The two copper atoms of the CuA site and the copper atom at heme a3 are smaller than the iron atoms in heme a and heme a3.
Similar articles
- The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation.
Bender E, Kadenbach B. Bender E, et al. FEBS Lett. 2000 Jan 21;466(1):130-4. doi: 10.1016/s0014-5793(99)01773-1. FEBS Lett. 2000. PMID: 10648827 - cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity.
Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. Lee I, et al. J Biol Chem. 2005 Feb 18;280(7):6094-100. doi: 10.1074/jbc.M411335200. Epub 2004 Nov 19. J Biol Chem. 2005. PMID: 15557277 - Cytochrome c oxidase from eucaryotes but not from procaryotes is allosterically inhibited by ATP.
Follmann K, Arnold S, Ferguson-Miller S, Kadenbach B. Follmann K, et al. Biochem Mol Biol Int. 1998 Aug;45(5):1047-55. doi: 10.1002/iub.7510450522. Biochem Mol Biol Int. 1998. PMID: 9739469 - Individual biochemical behaviour versus biological robustness: spotlight on the regulation of cytochrome c oxidase.
Ramzan R, Weber P, Kadenbach B, Vogt S. Ramzan R, et al. Adv Exp Med Biol. 2012;748:265-81. doi: 10.1007/978-1-4614-3573-0_11. Adv Exp Med Biol. 2012. PMID: 22729862 Review. - Regulation of cytochrome c oxidase by adenylic nucleotides. Is oxidative phosphorylation feedback regulated by its end-products?
Beauvoit B, Rigoulet M. Beauvoit B, et al. IUBMB Life. 2001 Sep-Nov;52(3-5):143-52. doi: 10.1080/152165401317316545. IUBMB Life. 2001. PMID: 11798026 Review.
Cited by
- Ibrutinib Promotes Atrial Fibrillation by Disrupting A-Kinase Anchoring Protein 1-Mediated Mitochondrial Quality Surveillance in Cardiomyocytes.
Li Y, Liu X, Lin R, Peng X, Wang X, Meng F, Jin S, Lv W, Liu X, Du Z, Wen S, Bai R, Ruan Y, Zhou H, Zou R, Tang R, Liu N. Li Y, et al. Research (Wash D C). 2024 Oct 29;7:0509. doi: 10.34133/research.0509. eCollection 2024. Research (Wash D C). 2024. PMID: 39469220 Free PMC article. - Mitochondrial CB₁ receptors regulate neuronal energy metabolism.
Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutiérrez S, Martín-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, López-Rodríguez ML, Grandes P, Rossignol R, Marsicano G. Bénard G, et al. Nat Neurosci. 2012 Mar 4;15(4):558-64. doi: 10.1038/nn.3053. Nat Neurosci. 2012. PMID: 22388959 - Mitochondria in heart failure.
Rosca MG, Hoppel CL. Rosca MG, et al. Cardiovasc Res. 2010 Oct 1;88(1):40-50. doi: 10.1093/cvr/cvq240. Epub 2010 Jul 28. Cardiovasc Res. 2010. PMID: 20668004 Free PMC article. Review. - Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway.
Bouchez C, Devin A. Bouchez C, et al. Cells. 2019 Mar 27;8(4):287. doi: 10.3390/cells8040287. Cells. 2019. PMID: 30934711 Free PMC article. Review. - Coenzyme q and the respiratory chain: coenzyme q pool and mitochondrial supercomplexes.
Enriquez JA, Lenaz G. Enriquez JA, et al. Mol Syndromol. 2014 Jul;5(3-4):119-40. doi: 10.1159/000363364. Mol Syndromol. 2014. PMID: 25126045 Free PMC article.
References
- Papa, S., Sardanelli, A. M., Scacco, S., and Technikova-Dobrova, Z. ( 1999) cAMP-dependent protein kinase and phosphoproteins in mammalian mitochondria. An extension of the cAMP-mediated intracellular signal transduction. FEBS Lett. 444, 245–249 - PubMed
- Goldenthal, M. J., and Marín-García, J. ( 2004) Mitochondrial signaling pathways: a receiver/integrator organelle. Mol. Cell. Biochem. 262, 1–16 - PubMed
- Horbinski, C., and Chu, C. T. ( 2005) Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic. Biol. Med. 38, 2–11 - PubMed
- Sardanelli, A. M., Signorile, A., Nuzzi, R., Rasmo, D. D., Technikova-Dobrova, Z., Drahot, Z., Occhiell, A., Pica, A., and Papa, S. ( 2006) Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 580, 5690–5696 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases