Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids - PubMed (original) (raw)
Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids
Lili Yang et al. J Biol Chem. 2008.
Abstract
We conducted a study coupling metabolomics and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. Rat livers were perfused with lactate or pyruvate +/- aminooxyacetate or mercaptopicolinate in the presence of 40% enriched NaH(13)CO(3). Other livers were perfused with dimethyl [1,4-(13)C(2)]succinate +/- mercaptopicolinate. In this first of two companion articles, we show that a substantial fraction of gluconeogenic carbon leaves the liver as citric acid cycle intermediates, mostly alpha-ketoglutarate. The efflux of gluconeogenic carbon ranges from 10 to 200% of the rate of liver gluconeogenesis. This cataplerotic efflux of gluconeogenic carbon may contribute to renal gluconeogenesis in vivo. Multiple crossover analyses of concentrations of gluconeogenic intermediates and redox measurements expand previous reports on the regulation of gluconeogenesis and the effects of inhibitors. We also demonstrate the formation of adducts from the condensation, in the liver, of (i) aminooxyacetate with pyruvate, alpha-ketoglutarate, and oxaloacetate and (ii) mercaptopicolinate and pyruvate. These adducts may exert metabolic effects unrelated to their effect on gluconeogenesis.
Figures
FIGURE 1.
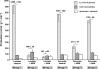
Glucose production (grey bars, in C3 units), release of citric acid cycle intermediates (hatched bars), and release of glutamate + aspartate (white bars) in livers were perfused with buffer containing 40% NaH13CO3, 5 mm lactate, or 2 mm pyruvate ± 0.3 mm mercaptopicolinate, or ± 0.5 mm aminooxyacetate. The numbers above each pair of grey and hatched bars represent a minimal rate of pyruvate carboxylation. Data are presented as mean ± S.E. (n = 4–7).
FIGURE 2.
Glucose production (grey bars, in C3 units), release of citric acid cycle intermediates (hatched bars), and release of glutamate + aspartate (white bars) in livers were perfused with buffer containing [1,4-13C2]succinate ± 0.3 mm mercaptopicolinate. Data are presented as mean ± S.E. (n = 4–6).
FIGURE 3.
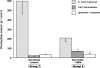
Relative redox ratios [αGP]/[DHAP] (hatched bars) and [malate]/[OAA] (white bars) in perfused livers. The ratios, calculated as described under “Experimental Procedures,” are expressed relative to the corresponding ratio of the lactate control group set to 100%.
FIGURE 4.
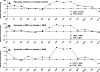
Crossover plots comparing the concentration of gluconeogenic and citric acid cycle intermediates in livers perfused with 2 mm pyruvate_versus_ 5 mm lactate. A, base-line conditions.B, +0.3 m
m
MPA. C, +0.5 m
m
AOA.C was redrawn with modifications from Fig. 3 of Ref. . CIT, citrate;MAL, malate; SUC, succinate; ASP, aspartate;2PG, 2-P-glycerate; 3PG, 3-P-glycerate; GLYC, glycerate; LACT, lactate.
FIGURE 5.
Crossover plots comparing the effects of 0.3 mm MPA or 0.5 mm AOA on the concentration of gluconeogenic and citric acid cycle intermediates in livers perfused with 5 mm lactate versus 2 mm pyruvate. A, lactate + MPA versus lactate. B, lactate + AOA versus lactate. C, pyruvate (PYR) + MPA versus pyruvate. D, pyruvate + AOA versus pyruvate. CIT, citrate; MAL, malate;SUC, succinate; ASP, aspartate; 2PG, 2-P-glycerate;3PG, 3-P-glycerate; GLYC, glycerate; LACT, lactate.
FIGURE 6.
Crossover plot showing the effects of 0.3 mm MPA on the concentration of gluconeogenic and citric acid cycle intermediates in livers perfused with dimethyl [1,4-13C2]succinate. CIT, citrate; MAL, malate; SUC, succinate;ASP, aspartate; 2PG, 2-P-glycerate; 3PG, 3-P-glycerate; GLYC, glycerate; PYR, pyruvate; α_KG_, α-ketoglutarate.
FIGURE 7.
In vitro formation of adducts between (i) AOA and either pyruvate (PYR), α-ketoglutarate (α_KG_), or OAA (A–I), and (ii) MPA and pyruvate (J–L). Each_vertical set_ of panels corresponds to the reaction of one ketoacid with either AOA or MPA. For example, a solution of 0.5 m
m
AOA (pH 7.4; T = 37 °C) was infused in the source of the mass spectrometer. After 1 min, 0.2 m
m
pyruvate was added to the AOA solution. The signals of AOA, pyruvate, and the pyruvate-AOA adduct are shown in C, B, and A, respectively.
Similar articles
- Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle: II. Heterogeneity of metabolite labeling pattern.
Yang L, Kasumov T, Kombu RS, Zhu SH, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Yang L, et al. J Biol Chem. 2008 Aug 8;283(32):21988-96. doi: 10.1074/jbc.M803455200. Epub 2008 Jun 10. J Biol Chem. 2008. PMID: 18544526 Free PMC article. - Isotopomer studies of gluconeogenesis and the Krebs cycle with 13C-labeled lactate.
Katz J, Wals P, Lee WN. Katz J, et al. J Biol Chem. 1993 Dec 5;268(34):25509-21. J Biol Chem. 1993. PMID: 7902352 - Rates of gluconeogenesis and citric acid cycle in perfused livers, assessed from the mass spectrometric assay of the 13C labeling pattern of glutamate.
Di Donato L, Des Rosiers C, Montgomery JA, David F, Garneau M, Brunengraber H. Di Donato L, et al. J Biol Chem. 1993 Feb 25;268(6):4170-80. J Biol Chem. 1993. PMID: 8095046 - Use of labeling pattern of liver glutamate to calculate rates of citric acid cycle and gluconeogenesis.
Large V, Brunengraber H, Odeon M, Beylot M. Large V, et al. Am J Physiol. 1997 Jan;272(1 Pt 1):E51-8. doi: 10.1152/ajpendo.1997.272.1.E51. Am J Physiol. 1997. PMID: 9038851
Cited by
- Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle: II. Heterogeneity of metabolite labeling pattern.
Yang L, Kasumov T, Kombu RS, Zhu SH, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Yang L, et al. J Biol Chem. 2008 Aug 8;283(32):21988-96. doi: 10.1074/jbc.M803455200. Epub 2008 Jun 10. J Biol Chem. 2008. PMID: 18544526 Free PMC article. - Retrograde signaling by a mtDNA-encoded non-coding RNA preserves mitochondrial bioenergetics.
Blumental-Perry A, Jobava R, Bederman I, Degar AJ, Kenche H, Guan BJ, Pandit K, Perry NA, Molyneaux ND, Wu J, Prendergas E, Ye ZW, Zhang J, Nelson CE, Ahangari F, Krokowski D, Guttentag SH, Linden PA, Townsend DM, Miron A, Kang MJ, Kaminski N, Perry Y, Hatzoglou M. Blumental-Perry A, et al. Commun Biol. 2020 Oct 30;3(1):626. doi: 10.1038/s42003-020-01322-4. Commun Biol. 2020. PMID: 33127975 Free PMC article. - Exploring the Process of Energy Generation in Pathophysiology by Targeted Metabolomics: Performance of a Simple and Quantitative Method.
Riera-Borrull M, Rodríguez-Gallego E, Hernández-Aguilera A, Luciano F, Ras R, Cuyàs E, Camps J, Segura-Carretero A, Menendez JA, Joven J, Fernández-Arroyo S. Riera-Borrull M, et al. J Am Soc Mass Spectrom. 2016 Jan;27(1):168-77. doi: 10.1007/s13361-015-1262-3. Epub 2015 Sep 17. J Am Soc Mass Spectrom. 2016. PMID: 26383735 - Stable isotope-resolved metabolomics and applications for drug development.
Fan TW, Lorkiewicz PK, Sellers K, Moseley HN, Higashi RM, Lane AN. Fan TW, et al. Pharmacol Ther. 2012 Mar;133(3):366-91. doi: 10.1016/j.pharmthera.2011.12.007. Epub 2011 Dec 23. Pharmacol Ther. 2012. PMID: 22212615 Free PMC article. Review. - Macrophages with a deletion of the phosphoenolpyruvate carboxykinase 1 (Pck1) gene have a more proinflammatory phenotype.
Ko CW, Counihan D, Wu J, Hatzoglou M, Puchowicz MA, Croniger CM. Ko CW, et al. J Biol Chem. 2018 Mar 2;293(9):3399-3409. doi: 10.1074/jbc.M117.819136. Epub 2018 Jan 9. J Biol Chem. 2018. PMID: 29317502 Free PMC article.
References
- Soga, T., Baran, R., Suematsu, M., Ueno, Y., Ikeda, S., Sakurakawa, T., Kakazu, Y., Ishikawa, T., Robert, M., Nishioka, T., and Tomita, M. J. (2006) J. Biol. Chem. 281 16768–16776 - PubMed
- Kell, D. B., and Oliver, S. G. (2004) BioEssays 26 99–105 - PubMed
- Rochfort, S. (2005) J. Nat. Prod. 68 1813–1820 - PubMed
- Weckwerth, W., and Morgenthal, K. (2005) Drug Discov. Today 10 1551–1558 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources