Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice - PubMed (original) (raw)
Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice
Natee Jearawiriyapaisarn et al. Mol Ther. 2008 Sep.
Abstract
Cell-penetrating peptides (CPPs), containing arginine (R), 6-aminohexanoic acid (X), and/or beta-alanine (B) conjugated to phosphorodiamidate morpholino oligomers (PMOs), enhance their delivery in cell culture. In this study, the potency, functional biodistribution, and toxicity of these conjugates were evaluated in vivo, in EGFP-654 transgenic mice that ubiquitously express the aberrantly spliced EGFP-654 pre-mRNA reporter. Correct splicing and enhanced green fluorescence protein (EGFP) upregulation serve as a positive readout for peptide-PMO (PPMO) entry into cells and access to EGFP-654 pre-mRNA in the nucleus. Intraperitoneal injections of a series of PPMOs, A-N (12 mg/kg), administered once a day for four successive days resulted in splicing correction in numerous tissues. PPMO-B was highly potent in the heart, diaphragm, and quadriceps, which are key muscles in the treatment of Duchenne muscular dystrophy. We therefore investigated PPMO M23D-B, designed to force skipping of stop-codon containing dystrophin exon 23, in an mdx mouse model of the disease. Systemic delivery of M23D-B yielded persistent exon 23 skipping, yielding high and sustained dystrophin protein expression in body-wide muscles, including cardiac muscle, without detectable toxicity. The rescued dystrophin reduced serum creatinine kinase to near-wild-type levels, indicating improvement in muscle integrity. This is the first report of oligonucleotide-mediated exon skipping and dystrophin protein induction in the heart of treated animals.
Figures
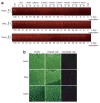
Figure 1. Correction of aberrant splicing in EGFP-654 mice by PPMO-654 conjugates, and the toxicity of the conjugates
(a) The EGFP-654 mice express a modified EGFP-654 pre-mRNA in which the enhanced green fluorescence protein (EGFP) coding region (boxes) is interrupted by an aberrantly spliced human β-globin IVS2-654 intron (line). The aberrantly spliced mRNA retains an intron fragment (red line), preventing proper translation of EGFP. Blocking the aberrant 5′-splice site with PPMO-654 (shaded bar) restores the correct splicing, leading to EGFP upregulation. The EGFP upregulation in mice is observed using a fluorescent live imaging system. (b) Reverse transcriptase-PCR (RT-PCR) of total RNA from the tissues of PPMO-treated EGFP-654 mice. The EGFP-654 mice were treated with PPMO-654 conjugates by intraperitoneal injection once every day for 4 days at 12 mg/kg/day. RNA samples were extracted from the indicated tissues of the treated mice and analyzed using RT-PCR 1 day after the last injection. The Cy5-labeled RT-PCR products from aberrantly spliced and correctly spliced EGFP mRNA are shown as Ab [160 base pairs (bp)] and C (87 bp), respectively. (c) Levels of serum aspartate aminotransferase and alanine aminotransferase were monitored before the treatment and at 1 day after the last injection of PPMO-654 conjugates. PPMO, peptide-phosphorodiamidate morpholino oligomer.
Figure 2. Effective and persistent splicing correction of PPMO-B conjugate in the heart, diaphragm (diap), and quadriceps (quad) of EGFP-654 mice
(a–b) Dose-dependent splicing correction. EGFP-654 mice were treated with PPMO-B conjugate at the indicated doses by intravenous (IV) injection once every day for 4 days. (a) Reverse transcriptase-PCR (RT-PCR) of total RNA and (b) fluorescent images of tissue sections were analyzed 1 day after the last injection. Samples A and B are from different mice. (Scale bar = 100 μm). (c–d) Time-dependent splicing correction. The EGFP-654 mice were injected IV with PPMO-B conjugate at 12 mg/kg/day for 4 days. (c) Total RNA samples from the heart, diap, and quad were analyzed using RT-PCR at the indicated time points. (d) Upregulation of enhanced green fluorescence protein (EGFP) expression was monitored using a fluorescent live imaging system. WT is a mouse that constitutively expresses EGFP. The EGFP-654 mice treated with PPMO-B, the control mice treated with PPMO-B at 12 mg/kg, and untreated mice are shown as B, scr-B, and un, respectively. PPMO, peptide-phosphorodiamidate morpholino oligomer.
Figure 3. Persistence of M23D-B-induced exon 23 skipping of dystrophin transcript in the heart, diaphragm (diap), and quadriceps (quad) of mdx mice after intravenous injection at 12 mg/kg/day for 4 days
RNA samples were analyzed using nested reverse transcriptase-PCR (RT-PCR) at the indicated time points. The nested RT-PCR product from the full-length dystrophin transcript is 445 base pairs (bp), indicated by boxes numbered 21–24. The product from exon 23–skipped mRNA is 232 bp, as indicated by boxes numbered 21, 22, and 24.
Figure 4. Restoration of dystrophin (Dys) expression in the heart, diaphragm (diap), and quadriceps (quad) of M23D-B-treated mice after intravenous injections at 12 mg/kg/day for 4 days
(a) In-gel western blot detection of total protein extracted from injected mdx muscles at the indicated time points. Dys protein was detected by mouse monoclonal antibody against C-terminus of Dys, followed by IRDye 680 goat anti-mouse immunoglobulin G (IgG) antibody. The three different Dys bands represent naturally occurring variants or synthetic intermediates of Dys. (b) Immunofluorescence detection of Dys on frozen muscle sections from the heart, diap, and quad of treated mdx mice. Sections were immunostained for Dys with NCL-DYS2 and detected using biotinylated anti-mouse IgG and fluorescein avidin. Sections from the heart, diap, and quad were analyzed 3, 2, and 2 weeks after the last injection, respectively. Muscle sections from C57BL and untreated mdx mice were included as positive and negative controls, respectively. Scale bar, 100 μm.
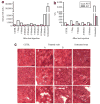
Figure 5. Improvement of pathology markers in mdx mice treated intravenously with M23D-B at 12 mg/kg/day for 4 days
(a) Serum creatinine kinase (CK). C57BL (n = 5), treated mdx mice (n = 2 at each time point), and untreated mdx mice (n = 20). (b) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). C57BL (n = 3), treated mdx mice (n = 2 at each time point), and untreated mdx mice (n = 10). All bar graphs represent mean except the bar graphs of serum CK of C57BL and untreated mdx mice, which represent mean and SEM. (c) Hematoxylin and eosin staining of cardiac muscle. C57BL at 8 weeks of age, treated mdx at 11 weeks of age (3 weeks after last injection), and untreated mdx at 8 weeks of age. Arrows, inflammatory infiltrate; Scale bar, 100 μm.
Comment in
- Peptide-conjugated antisense therapy takes a skip ahead.
Wells DJ. Wells DJ. Mol Ther. 2008 Sep;16(9):1523-4. doi: 10.1038/mt.2008.157. Mol Ther. 2008. PMID: 18725878 No abstract available.
Similar articles
- Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy.
Echigoya Y, Nakamura A, Nagata T, Urasawa N, Lim KRQ, Trieu N, Panesar D, Kuraoka M, Moulton HM, Saito T, Aoki Y, Iversen P, Sazani P, Kole R, Maruyama R, Partridge T, Takeda S, Yokota T. Echigoya Y, et al. Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):4213-4218. doi: 10.1073/pnas.1613203114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373570 Free PMC article. - Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice.
Desjardins CA, Yao M, Hall J, O'Donnell E, Venkatesan R, Spring S, Wen A, Hsia N, Shen P, Russo R, Lan B, Picariello T, Tang K, Weeden T, Zanotti S, Subramanian R, Ibraghimov-Beskrovnaya O. Desjardins CA, et al. Nucleic Acids Res. 2022 Nov 11;50(20):11401-11414. doi: 10.1093/nar/gkac641. Nucleic Acids Res. 2022. PMID: 35944903 Free PMC article. - Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy.
Moulton HM, Moulton JD. Moulton HM, et al. Biochim Biophys Acta. 2010 Dec;1798(12):2296-303. doi: 10.1016/j.bbamem.2010.02.012. Epub 2010 Feb 17. Biochim Biophys Acta. 2010. PMID: 20170628 - Cell-penetrating peptide-morpholino conjugates alter pre-mRNA splicing of DMD (Duchenne muscular dystrophy) and inhibit murine coronavirus replication in vivo.
Moulton HM, Fletcher S, Neuman BW, McClorey G, Stein DA, Abes S, Wilton SD, Buchmeier MJ, Lebleu B, Iversen PL. Moulton HM, et al. Biochem Soc Trans. 2007 Aug;35(Pt 4):826-8. doi: 10.1042/BST0350826. Biochem Soc Trans. 2007. PMID: 17635157 Review. - Screening for antisense modulation of dystrophin pre-mRNA splicing.
Dickson G, Hill V, Graham IR. Dickson G, et al. Neuromuscul Disord. 2002 Oct;12 Suppl 1:S67-70. doi: 10.1016/s0960-8966(02)00085-8. Neuromuscul Disord. 2002. PMID: 12206799 Review.
Cited by
- Systematic Review of Genetic Substrate Reduction Therapy in Lysosomal Storage Diseases: Opportunities, Challenges and Delivery Systems.
Beraza-Millor M, Rodríguez-Castejón J, Del Pozo-Rodríguez A, Rodríguez-Gascón A, Solinís MÁ. Beraza-Millor M, et al. BioDrugs. 2024 Sep;38(5):657-680. doi: 10.1007/s40259-024-00674-1. Epub 2024 Aug 23. BioDrugs. 2024. PMID: 39177875 Free PMC article. - Peptide-mediated Cell and In Vivo Delivery of Antisense Oligonucleotides and siRNA.
Järver P, Coursindel T, Andaloussi SE, Godfrey C, Wood MJ, Gait MJ. Järver P, et al. Mol Ther Nucleic Acids. 2012 Jun 12;1(6):e27. doi: 10.1038/mtna.2012.18. Mol Ther Nucleic Acids. 2012. PMID: 23344079 Free PMC article. No abstract available. - Dendritic Guanidines as Efficient Analogues of Cell Penetrating Peptides.
Bonduelle CV, Gillies ER. Bonduelle CV, et al. Pharmaceuticals (Basel). 2010 Mar 12;3(3):636-666. doi: 10.3390/ph3030636. Pharmaceuticals (Basel). 2010. PMID: 27713272 Free PMC article. Review. - Anti-tumor activity of splice-switching oligonucleotides.
Bauman JA, Li SD, Yang A, Huang L, Kole R. Bauman JA, et al. Nucleic Acids Res. 2010 Dec;38(22):8348-56. doi: 10.1093/nar/gkq731. Epub 2010 Aug 18. Nucleic Acids Res. 2010. PMID: 20719743 Free PMC article. - Therapeutic potential of splice-switching oligonucleotides.
Bauman J, Jearawiriyapaisarn N, Kole R. Bauman J, et al. Oligonucleotides. 2009 Mar;19(1):1-13. doi: 10.1089/oli.2008.0161. Oligonucleotides. 2009. PMID: 19125639 Free PMC article. Review.
References
- Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–1644. - PubMed
- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. - PubMed
- Arora V, Devi GR, Iversen PL. Neutrally charged phosphorodiamidate morpholino antisense oligomers: uptake, efficacy and pharmacokinetics. Curr Pharm Biotechnol. 2004;5:431–439. - PubMed
- Sazani P, Gemignani F, Kang SH, Maier MA, Manoharan M, Persmark M, et al. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat Biotechnol. 2002;20:1228–1233. - PubMed
- Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD, Iversen PL, et al. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem Soc Trans. 2007;35(Pt 4):775–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources