Shape induced inhibition of phagocytosis of polymer particles - PubMed (original) (raw)
Shape induced inhibition of phagocytosis of polymer particles
Julie A Champion et al. Pharm Res. 2009 Jan.
Abstract
Purpose: To determine if particle shape can be engineered to inhibit phagocytosis of drug delivery particles by macrophages, which can be a significant barrier to successful therapeutic delivery.
Methods: Non-spherical polystyrene particles were fabricated by stretching spherical particles embedded in a polymer film. A rat alveolar macrophage cell line was used as model macrophages. Phagocytosis of particles was assessed using time-lapse video microscopy and fluorescence microscopy.
Results: We fabricated worm-like particles with very high aspect ratios (>20). This shape exhibits negligible phagocytosis compared to conventional spherical particles of equal volume. Reduced phagocytosis is a result of decreasing high curvature regions of the particle to two single points, the ends of the worm-like particles. Internalization is possible only at these points, while attachment anywhere along the length of the particles inhibits internalization due to the low curvature.
Conclusions: Shape-induced inhibition of phagocytosis of drug delivery particles is possible by minimizing the size-normalized curvature of particles. We have created a high aspect ratio shape that exhibits negligible uptake by macrophages.
Figures
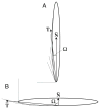
Fig. 1
A schematic diagram illustrating how local shape is defined. T̄ represents the average of tangential angles near the point of cell contact. Ω is the angle between T̄ and the membrane normal at the site of attachment, N̄. A _Ω_=2.5° for cell attachment at end of worm. B _Ω_=87.5° for cell attachment on side of worm.
Fig. 2
Scanning electron micrographs of polystyrene A worms stretched from 3 μm spheres and B worms stretched from 1 μm spheres. Scale bars = 10 μm (A) and 2 μm (B).
Fig. 3
Time-lapse video microscopy clips at 0, 1, 2, 3, 4 and 32 min after attachment of IgG-adsorbed PS particles to alveolar macrophages. A Macrophage internalizes 3 μm sphere quickly following attachment. B Macrophage attaches to side of worm, but does not internalize it. Images have been cropped and magnified identically to show cell/particle detail. Scale bars = 5 μm.
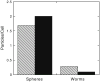
Fig. 4
Average number of spherical and worm-like IgG-adsorbed particles internalized in 22 h as determined by fluorescence microscopy. Patterned bars indicate particle volumes equivalent to 3 μm spheres (p<0.0000031) and solid bars indicate particle volumes equivalent to 1 μm spheres (p<0.000024). At least 50 cells were counted for each sample.
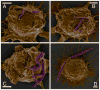
Fig. 5
Colored scanning electron micrographs demonstrate the flexibility of IgG-adsorbed (A–C 1μm volume; D 3 μm volume) worm-like particles and ability of macrophages to bend worms by attachment at different points. Scale bars = 2 μm.
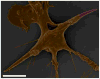
Fig. 6
Colored scanning electron micrograph shows macrophage internalizing an IgG-adsorbed worm (3 μm volume) and the effect of internalization on cell shape. Scale bar = 5 μm.
Similar articles
- Loading PEG-catalase into filamentous and spherical polymer nanocarriers.
Simone EA, Dziubla TD, Arguiri E, Vardon V, Shuvaev VV, Christofidou-Solomidou M, Muzykantov VR. Simone EA, et al. Pharm Res. 2009 Jan;26(1):250-60. doi: 10.1007/s11095-008-9744-7. Epub 2008 Oct 28. Pharm Res. 2009. PMID: 18956141 Free PMC article. - Influence of particle geometry and PEGylation on phagocytosis of particulate carriers.
Mathaes R, Winter G, Besheer A, Engert J. Mathaes R, et al. Int J Pharm. 2014 Apr 25;465(1-2):159-64. doi: 10.1016/j.ijpharm.2014.02.037. Epub 2014 Feb 21. Int J Pharm. 2014. PMID: 24560647 - Superior transfection efficiency of phagocytic astrocytes by large chitosan/DNA nanoparticles.
Kong F, Liu G, Zhou S, Guo J, Chen S, Wang Z. Kong F, et al. Int J Biol Macromol. 2017 Dec;105(Pt 2):1473-1481. doi: 10.1016/j.ijbiomac.2017.06.061. Epub 2017 Jun 12. Int J Biol Macromol. 2017. PMID: 28619643 - Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): effect of poly(ethylene glycol) grafting density.
Essa S, Rabanel JM, Hildgen P. Essa S, et al. Int J Pharm. 2011 Jun 15;411(1-2):178-87. doi: 10.1016/j.ijpharm.2011.02.039. Epub 2011 Mar 31. Int J Pharm. 2011. PMID: 21458551 - Non-spherical micro- and nanoparticles: fabrication, characterization and drug delivery applications.
Mathaes R, Winter G, Besheer A, Engert J. Mathaes R, et al. Expert Opin Drug Deliv. 2015 Mar;12(3):481-92. doi: 10.1517/17425247.2015.963055. Epub 2014 Oct 18. Expert Opin Drug Deliv. 2015. PMID: 25327886 Review.
Cited by
- Exploring the influence of silicon oxide microchips shape on cellular uptake using imaging flow cytometry.
Bruce G, Bagherpour S, Duch M, Plaza JA, Stolnik S, Pérez-García L. Bruce G, et al. Mikrochim Acta. 2024 Aug 21;191(9):554. doi: 10.1007/s00604-024-06631-7. Mikrochim Acta. 2024. PMID: 39168870 Free PMC article. - Role of particle local curvature in cellular wrapping.
Khosravanizadeh A, Sens P, Mohammad-Rafiee F. Khosravanizadeh A, et al. J R Soc Interface. 2022 Nov;19(196):20220462. doi: 10.1098/rsif.2022.0462. Epub 2022 Nov 2. J R Soc Interface. 2022. PMID: 36321371 Free PMC article. - Higher lung accumulation of intravenously injected organic nanotubes.
Maitani Y, Nakamura Y, Kon M, Sanada E, Sumiyoshi K, Fujine N, Asakawa M, Kogiso M, Shimizu T. Maitani Y, et al. Int J Nanomedicine. 2013;8:315-23. doi: 10.2147/IJN.S38462. Epub 2013 Jan 18. Int J Nanomedicine. 2013. PMID: 23345977 Free PMC article. - Shape Control in Engineering of Polymeric Nanoparticles for Therapeutic Delivery.
Williford JM, Santos JL, Shyam R, Mao HQ. Williford JM, et al. Biomater Sci. 2015 Jul;3(7):894-907. doi: 10.1039/C5BM00006H. Biomater Sci. 2015. PMID: 26146550 Free PMC article. - Injectable, Ribbon-Like Microconfetti Biopolymer Platform for Vaccine Applications.
Moore KM, Batty CJ, Stiepel RT, Genito CJ, Bachelder EM, Ainslie KM. Moore KM, et al. ACS Appl Mater Interfaces. 2020 Sep 2;12(35):38950-38961. doi: 10.1021/acsami.0c10276. Epub 2020 Aug 24. ACS Appl Mater Interfaces. 2020. PMID: 32805875 Free PMC article.
References
- Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. - PubMed
- Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp Cell Res. 1998;242:265–273. - PubMed
- Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: Does size matter. Methods. 2006;40:1–9. - PubMed
- Illum L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood clearance and organ deposition of intravenously administered colloidal particles—the effects of particle size, nature and shape. Int J Pharm. 1982;12:135–146.
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharm Rev. 2001;53:283–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources