Arachidonic acid and ion channels: an update - PubMed (original) (raw)
Review
Arachidonic acid and ion channels: an update
H Meves. Br J Pharmacol. 2008 Sep.
Abstract
Arachidonic acid (AA), a polyunsaturated fatty acid with four double bonds, has multiple actions on living cells. Many of these effects are mediated by an action of AA or its metabolites on ion channels. During the last 10 years, new types of ion channels, transient receptor potential (TRP) channels, store-operated calcium entry (SOCE) channels and non-SOCE channels have been studied. This review summarizes our current knowledge about the effects of AA on TRP and non-SOCE channels as well as classical ion channels. It aims to distinguish between effects of AA itself and effects of AA metabolites. Lipid mediators are of clinical interest because some of them (for example, leukotrienes) play a role in various diseases, others (such as prostaglandins) are targets for pharmacological therapeutic intervention.
Figures
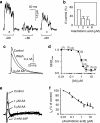
Figure 1
Effects of low (<10 μ
M
) concentrations of arachidonic acid on A-type transient K+ currents. (a) The transient A-type current expressed by Kv4.2 in Xenopus oocytes is inhibited by 4 μ
M
AA; the figure shows averaged records from an inside-out macropatch subjected to five depolarizations from −80 to +80 mV in the control (curve a), in 4 μ
M
AA (curve b) and after wash (curve c). (b) Current amplitude in percent of control at four concentrations of AA (a and b from Villarroel and Schwarz, 1996). (c) Inhibition of the A-type transient K+ current of hippocampal pyramidal neurons by 3 μ
M
AA; test pulse to +40 mV following prepulse to −110 mV; τ of inactivation is 35 ms in control and 2.6 ms in 3 μ
M
AA. (d) Dose-response curve for the effect of AA on the charge transfer associated with the transient current; the numbers of measurements are given in brackets. (c and d from Keros and McBain, 1997). (e) AA (1 and 5 μ
M
) reduces the amplitude of the A-type current of a hippocampal pyramidal cell; the current is fully blocked by 2 m
M
4AP; test pulse to +30 mV following prepulse to −120 mV. (f) Average dose-response curve (_n_=4–6) showing that the EC50 for AA is 0.27 μ
M
(e and f from Ramakers and Storm, 2002). AA, arachidonic acid.
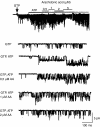
Figure 2
Polyunsaturated fatty acids accelerate the inactivation of the delayed rectifier current. (a) Records from a rat pulmonary arterial myocyte in control and 1, 2 and 3 min after application of 10 μ
M
arachidonic acid. (b) Records from hKv1.5 channels expressed in CHO cells in control and after application of 10 μ
M
AA. (c) Records from a NG108-15 neuroblastoma × glioma cell in control and in the presence of 5 μ
M
stearic acid (18:0), oleic acid (18:1) and linoleic acid (18:2); peak current in control and stearic acid 3.2 nA; currents in oleic and linoleic acid multiplied by 1.8 and 1.2, respectively. (d) Effect of 4 μ
M
AA on delayed rectifier channels expressed in Xenopus oocytes; AA applied to the cytoplasmic side of the membrane; currents recorded at 2 s intervals; scale bar 1 nA. Pulse potential +60 mV in panel a, +20 mV in panel b, +50 mV in panel c. Holding potential −60 mV in panel a, −100 mV in panel b, −80 mV in panels c and d. Experiments were carried out at room temperature. Panel a from Smirnow and Aaronson, 1996; panel b from Gavrilova-Ruch et al., 2007; panel c from Rouzaire-Dubois et al., 1991 and panel d from Oliver et al. (2004). AA, arachidonic acid.
Figure 3
Inhibitory effect of small AA concentrations on the KAch channel. Native KAch channels in rat atrial cells are activated by 100 μ
M
GTP and 4 m
M
ATP in an inside-out patch. AA, applied in stepwise increased concentration, inhibits channel activity (Kim and Pleumsamran, 2000). AA, arachidonic acid.
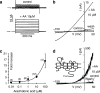
Figure 4
Activation of TRAAK in COS cells. (a) Whole-cell currents elicited by voltage pulses ranging from −130 to +50 mV in 20 mV steps from a holding potential of −80 mV before and after 2 min perfusion with 10 μ
M
AA. (b) Current-voltage curves obtained with voltage ramps (from −150 to +50 mV in 0.5 s) in control, after 3 min perfusion with 10 μ
M
AA and after wash. (c) Dose-response curve for the current at +50 mV (relative to control) with number of tested cells in brackets. (d) Current-voltage curves obtained from inside-out patch with voltage ramps (from −150 to +50 mV in 0.5 s) in control, after 3 min perfusion with 10 μ
M
AA and after wash (a–d from Fink et al., 1998). Inset in panel d shows membrane topology of TRAAK (Kim et al., 2001a). AA, arachidonic acid; TRAAK, TWIK-related AA-stimulated K+ channel. COS is a fibroblast cell line from the kidney of the African green monkey.
Figure 5
TRP channels are targets of fatty acids and AA metabolites. (a) A unit of 20 μ
M
linoleic acid repeatedly activates light-sensitive TRP channels of Drosophila; whole-cell record at −70 mV in the dark (Chyb et al., 1999). (b) Lipoxygenase products and anandamide activate the capsaicin-activated channel TRPV1 in isolated membrane patches of rat dorsal root neurons; channel activity (NP0) was calculated as the product of functional channels (N) in the patch and channel open probability (P0) (Hwang et al., 2000). (c) Activation of TRPV4 channels in HEK-293 cells by 5 μ
M
5′,6′-epoxyeicosatrienoic acid (EET); top: whole-cell record at 0 mV; bottom: current-voltage curves in control (1) and EET (2) (Watanabe et al., 2003). (d) Activation of TRPV4-like non-selective cation channels in B lymphocytes by 2 μ
M
5′,6′-EET and blockage by 1 μ
M
RR; whole-cell record from a cell held at −80 mV (Liu et al., 2006). TRP, transient receptor potential; RR, ruthenium red.
Figure 6
Store-operated Ca2+ entry (SOCE) and arachidonate-activated Ca2+ entry (non-SOCE) in HEK-293 and Saos-2 cells. (a and b) In the absence of extracellular Ca2+ thapsigargin (a) and AA (b) evoke in HEK-293 cells a transient increase in [Ca2+]i; restoration of extracellular Ca2+ results in Ca2+ entry. (c and d) HEK-293 (c) and Saos-2 cells (d) were treated with thapsigargin in the presence of extracellular Ca2+; this resulted in Ca2+ release followed by a sustained plateau of [Ca2+]i (SOCE); the subsequent addition of AA evoked a rapid inhibition of SOCE and the gradual activation of an alternative form of Ca2+ entry (non-SOCE). (Holmes et al., 2007). AA, arachidonic acid.
Similar articles
- Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels.
Miura H, Gutterman DD. Miura H, et al. Circ Res. 1998 Sep 7;83(5):501-7. doi: 10.1161/01.res.83.5.501. Circ Res. 1998. PMID: 9734472 Clinical Trial. - New Aspects of the Contribution of ER to SOCE Regulation: TRPC Proteins as a Link Between Plasma Membrane Ion Transport and Intracellular Ca2+ Stores.
Bavencoffe A, Zhu MX, Tian JB. Bavencoffe A, et al. Adv Exp Med Biol. 2017;993:239-255. doi: 10.1007/978-3-319-57732-6_13. Adv Exp Med Biol. 2017. PMID: 28900918 Review. - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - Modulation of cardiac gap junctions: the mode of action of arachidonic acid.
Schmilinsky-Fluri G, Valiunas V, Willi M, Weingart R. Schmilinsky-Fluri G, et al. J Mol Cell Cardiol. 1997 Jun;29(6):1703-13. doi: 10.1006/jmcc.1997.0409. J Mol Cell Cardiol. 1997. PMID: 9220356 - Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils.
Itagaki K, Kannan KB, Singh BB, Hauser CJ. Itagaki K, et al. J Immunol. 2004 Jan 1;172(1):601-7. doi: 10.4049/jimmunol.172.1.601. J Immunol. 2004. PMID: 14688372
Cited by
- The endocannabinoid system in guarding against fear, anxiety and stress.
Lutz B, Marsicano G, Maldonado R, Hillard CJ. Lutz B, et al. Nat Rev Neurosci. 2015 Dec;16(12):705-18. doi: 10.1038/nrn4036. Nat Rev Neurosci. 2015. PMID: 26585799 Free PMC article. Review. - TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells.
Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M, Crandall A, Heurteaux C, Hartnett ME, Križaj D. Yarishkin O, et al. J Gen Physiol. 2018 Dec 3;150(12):1660-1675. doi: 10.1085/jgp.201812179. Epub 2018 Nov 16. J Gen Physiol. 2018. PMID: 30446509 Free PMC article. - Arachidonic acid hyperpolarizes mesenchymal stromal cells from the human adipose tissue by stimulating TREK1 K+ channels.
Tarasov MV, Kotova PD, Bystrova MF, Kabanova NV, Sysoeva VY, Kolesnikov SS. Tarasov MV, et al. Channels (Austin). 2019 Dec;13(1):36-47. doi: 10.1080/19336950.2019.1565251. Channels (Austin). 2019. PMID: 30661462 Free PMC article. - Modulation of T-type calcium channels by bioactive lipids.
Chemin J, Cazade M, Lory P. Chemin J, et al. Pflugers Arch. 2014 Apr;466(4):689-700. doi: 10.1007/s00424-014-1467-5. Epub 2014 Feb 16. Pflugers Arch. 2014. PMID: 24531745 Review. - Arachidonic acid, ARC channels, and Orai proteins.
Shuttleworth TJ. Shuttleworth TJ. Cell Calcium. 2009 Jun;45(6):602-10. doi: 10.1016/j.ceca.2009.02.001. Epub 2009 Mar 17. Cell Calcium. 2009. PMID: 19278724 Free PMC article. Review.
References
- Alicia S, Angélica Z, Carlos S, Alfonso S, Luis V. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: Moving TRPC1 in and out of lipid rafts. Cell Ca. 2008. - PubMed
- Alloisio S, Aiello R, Ferroni S, Nobile M. Potentiation of native and recombinant P2X7-mediated calcium signalling by arachidonic acid in cultured cortical astrocytes and human embryonic kidney 293 cells. Mol Pharmacol. 2006;69:1975–1983. - PubMed
- Andoh T, Kuraishi Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Mol Brain Res. 2005;137:263–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources