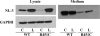Trafficking of cholinesterases and neuroligins mutant proteins. An association with autism - PubMed (original) (raw)
Trafficking of cholinesterases and neuroligins mutant proteins. An association with autism
Antonella De Jaco et al. Chem Biol Interact. 2008.
Abstract
Autism encompasses a wide spectrum of disorders arising during brain development. Recent studies reported that sequence polymorphisms in neuroligin-3 (NLGN3) and neuroligin-4 (NLGN4) genes have been linked to autism spectrum disorders indicating neuroligin genes as candidate targets in brain disorders. We have characterized a single mutation found in two affected brothers that substituted Arg451 to Cys in NL3. Our data show that the exposed Cys causes retention of the protein in the endoplasmic reticulum (ER) when expressed in HEK-293 cells. To examine whether the introduction of a Cys in the C-terminal region of other alpha/beta-hydrolase fold proteins could promote the same cellular phenotype, we made homologous mutations in acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) and found a similar processing deficiency and intracellular retention (De Jaco et al., J Biol Chem. 2006, 281:9667-76). NL3, AChE and BChE mutant proteins are recognized as misfolded in the ER, and degraded via the proteasome pathway. A 2D electrophoresis coupled with mass spectrometry based approach was used to analyze proteins co-immunoprecipitating with NL3 and show differential expression of factors interacting with wild type and mutant NL3. We identified several proteins belonging to distinct ER resident chaperones families, including calnexin, responsible for playing a role in the folding steps of the AChE and NLs.
Figures
Fig. 1
Western blot analysis of the expression levels of neuroligin in cells transfected with c-DNA for neuroligin-3 wild type and R451C mutant protein. C = control, L = lactacystin. (Lysate data are modified from Ref. [4].)
Fig. 2
2D SDS-PAGE gels of immunoprecipitated neuroligin-3 wild type and R451C mutant. Differences in associated proteins between neuroligin WT and neuroligin RC are shown by the arrows.
Similar articles
- A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha,beta-hydrolase fold protein family.
De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radic Z, Lockridge O, Ellisman MH, Taylor P. De Jaco A, et al. J Biol Chem. 2006 Apr 7;281(14):9667-76. doi: 10.1074/jbc.M510262200. Epub 2006 Jan 24. J Biol Chem. 2006. PMID: 16434405 - Folding anomalies of neuroligin3 caused by a mutation in the alpha/beta-hydrolase fold domain.
De Jaco A, Dubi N, Comoletti D, Taylor P. De Jaco A, et al. Chem Biol Interact. 2010 Sep 6;187(1-3):56-8. doi: 10.1016/j.cbi.2010.03.012. Epub 2010 Mar 12. Chem Biol Interact. 2010. PMID: 20227402 Free PMC article. - The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing.
Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. Comoletti D, et al. J Neurosci. 2004 May 19;24(20):4889-93. doi: 10.1523/JNEUROSCI.0468-04.2004. J Neurosci. 2004. PMID: 15152050 Free PMC article. - A review on the current neuroligin mouse models.
Xu JY, Xia QQ, Xia J. Xu JY, et al. Sheng Li Xue Bao. 2012 Oct 25;64(5):550-62. Sheng Li Xue Bao. 2012. PMID: 23090496 Review. - [Cholinesterases: anchored enzymes in membranes and basal laminae].
Krejci E. Krejci E. J Soc Biol. 2005;199(1):55-60. doi: 10.1051/jbio:2005007. J Soc Biol. 2005. PMID: 16114264 Review. French.
Cited by
- Roles of neuroligins in central nervous system development: focus on glial neuroligins and neuron neuroligins.
Liu X, Hua F, Yang D, Lin Y, Zhang L, Ying J, Sheng H, Wang X. Liu X, et al. J Transl Med. 2022 Sep 10;20(1):418. doi: 10.1186/s12967-022-03625-y. J Transl Med. 2022. PMID: 36088343 Free PMC article. Review. - Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology.
Di Jeso B, Arvan P. Di Jeso B, et al. Endocr Rev. 2016 Feb;37(1):2-36. doi: 10.1210/er.2015-1090. Epub 2015 Nov 23. Endocr Rev. 2016. PMID: 26595189 Free PMC article. Review. - Endosomal system genetics and autism spectrum disorders: A literature review.
Patak J, Zhang-James Y, Faraone SV. Patak J, et al. Neurosci Biobehav Rev. 2016 Jun;65:95-112. doi: 10.1016/j.neubiorev.2016.03.022. Epub 2016 Apr 2. Neurosci Biobehav Rev. 2016. PMID: 27048963 Free PMC article. Review. - Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer.
Wang X, Lee J, Di Jeso B, Treglia AS, Comoletti D, Dubi N, Taylor P, Arvan P. Wang X, et al. J Biol Chem. 2010 Jun 4;285(23):17564-73. doi: 10.1074/jbc.M110.111641. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353937 Free PMC article. - Ubiquitin-Proteasome System in Neurodegenerative Disorders.
Rao G, Croft B, Teng C, Awasthi V. Rao G, et al. J Drug Metab Toxicol. 2015;6(4):187. doi: 10.4172/2157-7609.1000187. Epub 2015 Aug 13. J Drug Metab Toxicol. 2015. PMID: 30761219 Free PMC article.
References
- Yen T, Nightingale BN, Burns JC, Sullivan DR, Stewart PM. Clin. Chem. 2003;49:1297–1308. - PubMed
- De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radić Z, Lockridge O, et al. J. Biol. Chem. 2006;281:9667–9676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P42 ES010337-099005/ES/NIEHS NIH HHS/United States
- GM 18360/GM/NIGMS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 GM018360/GM/NIGMS NIH HHS/United States
- P42 ES 10337/ES/NIEHS NIH HHS/United States
- R37 GM018360/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous