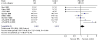Third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke - PubMed (original) (raw)
doi: 10.1186/1745-6215-9-37.
Richard Lindley, Joanna Wardlaw, Martin Dennis, Steff Lewis, Graham Venables, Adam Kobayashi, Anna Czlonkowska, Eivind Berge, Karsten Bruins Slot, Veronica Murray, Andre Peeters, Graeme Hankey, Karl Matz, Michael Brainin, Stefano Ricci, Maria Grazia Celani, Enrico Righetti, Teresa Cantisani, Gord Gubitz, Steve Phillips, Antonio Arauz, Kameshwar Prasad, Manuel Correia, Phillippe Lyrer; IST-3 Collaborative Group
Collaborators, Affiliations
- PMID: 18559104
- PMCID: PMC2442584
- DOI: 10.1186/1745-6215-9-37
Third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke
Peter Sandercock et al. Trials. 2008.
Abstract
Background: Intravenous recombinant tissue plasminogen activator (rt-PA) is approved for use in selected patients with ischaemic stroke within 3 hours of symptom onset. IST-3 seeks to determine whether a wider range of patients may benefit.
Design: International, multi-centre, prospective, randomized, open, blinded endpoint (PROBE) trial of intravenous rt-PA in acute ischaemic stroke. Suitable patients must be assessed and able to start treatment within 6 hours of developing symptoms, and brain imaging must have excluded intracerebral haemorrhage. With 1000 patients, the trial can detect a 7% absolute difference in the primary outcome. With 3500 patients, it can detect a 4.0% absolute benefit & with 6000, (mostly treated between 3 & 6 hours), it can detect a 3% benefit. TRIAL PROCEDURES: Patients are entered into the trial by telephoning a fast, secure computerised central randomisation system or via a secure web interface. Repeat brain imaging must be performed at 24-48 hours. The scans are reviewed 'blind' by expert readers. The primary measure of outcome is the proportion of patients alive and independent (Modified Rankin 0-2) at six months (assessed via a postal questionnaire mailed directly to the patient). Secondary outcomes include: events within 7 days (death, recurrent stroke, symptomatic intracranial haemorrhage), outcome at six months (death, functional status, EuroQol).
Trial registration: ISRCTN25765518.
Figures
Figure 1
Results of a systematic review of the randomised trials of thrombolysis with rt-PA in acute ischaemic stroke. Effects on death at the end of follow-up. The estimate of treatment effect for each trial is expressed as an odds ratio (solid square) and its 95% confidence interval (horizontal line). The size of the black square is proportional to the amount of information available. An odds ratio of 1.0 corresponds to a treatment effect of zero, an odds ratio of less than 1.0 suggests treatment is better than control, and an odds ratio of greater than 1.0 suggests treatment is worse than control. The overall result and its 95% CI is represented by a diamond. (from Wardlaw et al [9]). Copyright Cochrane Collaboration, reproduced with permission.)
Figure 2
The adjusted odds ratio of the chance of a favourable outcome (modified Rankin score of 0–1, Barthel Index 95–100, NIHSS 0–1) at day 90 following thrombolysis with rt-PA by stroke onset to treatment time, derived from an analysis of individual patient data from the main randomised trials of rt-PA in acute ischaemic stroke. (from rt-PA Study group investigators [10], copyright Elsevier and the Lancet, reproduced with permission).
Similar articles
- The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial.
IST-3 collaborative group; Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, Phillips SJ, Arauz A. IST-3 collaborative group, et al. Lancet. 2012 Jun 23;379(9834):2352-63. doi: 10.1016/S0140-6736(12)60768-5. Epub 2012 May 23. Lancet. 2012. PMID: 22632908 Free PMC article. Clinical Trial. - Imaging perfusion deficits, arterial patency and thrombolysis safety and efficacy in acute ischaemic stroke. An observational study of the effect of advanced imaging methods in The Third International Stroke Trial (IST-3), a randomised controlled trial.
Wardlaw JM, Carpenter T, Sakka E, Mair G, Cohen G, Shuler K, Palmer JM, Innes K, Sandercock PA. Wardlaw JM, et al. Southampton (UK): NIHR Journals Library; 2014 Jul. Southampton (UK): NIHR Journals Library; 2014 Jul. PMID: 25642519 Free Books & Documents. Review. - Update on the third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke and baseline features of the 3035 patients recruited.
Sandercock P, Lindley R, Wardlaw J, Dennis M, Innes K, Cohen G, Whiteley W, Perry D, Soosay V, Buchanan D, Venables G, Czlonkowska A, Kobayashi A, Berge E, Slot KB, Murray V, Peeters A, Hankey GJ, Matz K, Brainin M, Ricci S, Cantisani TA, Gubitz G, Phillips SJ, Antonio A, Correia M, Lyrer P, Kane I, Lundstrom E; IST-3 collaborative group. Sandercock P, et al. Trials. 2011 Nov 30;12:252. doi: 10.1186/1745-6215-12-252. Trials. 2011. PMID: 22129158 Free PMC article. Clinical Trial. - Thrombolysis for acute ischaemic stroke.
Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Wardlaw JM, et al. Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. PMID: 12917889 Updated. Review. - Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six-hours of acute ischemic stroke.
Wardlaw JM, von Kummer R, Carpenter T, Parsons M, Lindley RI, Cohen G, Murray V, Kobayashi A, Peeters A, Chappell F, Sandercock PA. Wardlaw JM, et al. Int J Stroke. 2015 Aug;10(6):956-68. doi: 10.1111/j.1747-4949.2012.00946.x. Epub 2013 Jan 22. Int J Stroke. 2015. PMID: 23336348 Clinical Trial.
Cited by
- Intravenous thrombolytic treatment in the oldest old.
García-Caldentey J, Alonso de Leciñana M, Simal P, Fuentes B, Reig G, Díaz-Otero F, Guillán M, García A, Martínez P, García-Pastor A, Egido JA, Díez-Tejedor E, Gil-Núñez A, Vivancos J, Masjuan J. García-Caldentey J, et al. Stroke Res Treat. 2012;2012:923676. doi: 10.1155/2012/923676. Epub 2012 Jul 16. Stroke Res Treat. 2012. PMID: 22848866 Free PMC article. - Successful thrombolysis for acute ischaemic stroke in haemodialysis.
Power A, Moser S, Duncan N. Power A, et al. NDT Plus. 2010 Dec;3(6):576-8. doi: 10.1093/ndtplus/sfq154. Epub 2010 Aug 24. NDT Plus. 2010. PMID: 25949472 Free PMC article. - Intravenous thrombolysis for acute cerebral ischaemia in old stroke patients ≥ 80 years of age.
Boulouis G, Dumont F, Cordonnier C, Bodenant M, Leys D, Hénon H. Boulouis G, et al. J Neurol. 2012 Jul;259(7):1461-7. doi: 10.1007/s00415-011-6359-4. Epub 2011 Dec 20. J Neurol. 2012. PMID: 22183776 - Improved in-hospital outcomes and care for patients in stroke research: An observational study.
Purvis T, Hill K, Kilkenny M, Andrew N, Cadilhac D. Purvis T, et al. Neurology. 2016 Jul 12;87(2):206-13. doi: 10.1212/WNL.0000000000002834. Epub 2016 Jun 15. Neurology. 2016. PMID: 27306625 Free PMC article. - Italian guidelines on thrombolysis indications in ischaemic stroke have been revised after IST 3 trial and Cochrane Review: PROS.
Ricci S, Cenciarelli S, Mazzoli T. Ricci S, et al. Intern Emerg Med. 2013 Dec;8(8):653-4. doi: 10.1007/s11739-013-0987-x. Epub 2013 Aug 23. Intern Emerg Med. 2013. PMID: 23975392 No abstract available.
References
- Murray CJL, Lopez AD. The Global Burden of Disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Boston: Harvard University Press; 1996.
- Asplund K. Stroke in Europe: widening gap between East and West. Cerebrovascular Diseases. 1996;6:3–6. doi: 10.1159/000107983. - DOI
- Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, Xie JX, Warlow C, Peto R. On behalf of the CAST and IST collaborative groups. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40 000 randomized patients from the Chinese acute stroke trial and the international stroke trial. Stroke. 2000;31:1240–9. - PubMed
LinkOut - more resources
Full Text Sources