The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction - PubMed (original) (raw)
The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction
David Varga-Szabo et al. J Exp Med. 2008.
Abstract
Platelet activation and aggregation are essential to limit posttraumatic blood loss at sites of vascular injury but also contributes to arterial thrombosis, leading to myocardial infarction and stroke. Agonist-induced elevation of [Ca(2+)](i) is a central step in platelet activation, but the underlying mechanisms are not fully understood. A major pathway for Ca(2+) entry in nonexcitable cells involves receptor-mediated release of intracellular Ca(2+) stores, followed by activation of store-operated calcium (SOC) channels in the plasma membrane. Stromal interaction molecule 1 (STIM1) has been identified as the Ca(2+) sensor in the endoplasmic reticulum (ER) that activates Ca(2+) release-activated channels in T cells, but its role in mammalian physiology is unknown. Platelets express high levels of STIM1, but its exact function has been elusive, because these cells lack a normal ER and Ca(2+) is stored in a tubular system referred to as the sarcoplasmatic reticulum. We report that mice lacking STIM1 display early postnatal lethality and growth retardation. STIM1-deficient platelets have a marked defect in agonist-induced Ca(2+) responses, and impaired activation and thrombus formation under flow in vitro. Importantly, mice with STIM1-deficient platelets are significantly protected from arterial thrombosis and ischemic brain infarction but have only a mild bleeding time prolongation. These results establish STIM1 as an important mediator in the pathogenesis of ischemic cardio- and cerebrovascular events.
Figures
Figure 1.
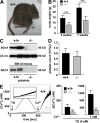
Defective SOCE in Stim1-deficient platelets. (A) 5-wk-old wild-type and Stim1−/− littermates. (B) Body weights of wild-type (+/+) and Stim1−/− (−/−) mice. Values are mean ± SD. ***, P < 0.001. (C) Western blot analyses of platelet lysates from mice with the indicated genotypes (top) or of mice transplanted with the indicated bone marrow (bottom). Stim1 was assessed using an antibody that can recognize the N-terminal region of the protein (GOK/Stim1; reference 11). An antibody to β3 integrin served as control. Results from two individuals per group are shown. (D) Peripheral platelet counts in wild-type and Stim1−/− mice. Values are mean ± SD. (E) Fura-2–loaded platelets were stimulated with 5 μM TG for 10 min, followed by the addition of extracellular Ca2+ and monitoring of [Ca2+]i. Representative measurements (left) and maximal increase in intracellular Ca2+ concentrations compared with baseline levels (Δ[Ca2+]i) ± SD (n = 4 mice per group) before and after addition of 1 mM Ca2+ (right) are shown. **, P < 0.01; ***, P < 0.001.
Figure 2.
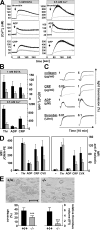
Defective agonist-induced Ca2+ signaling and aggregate formation under flow in Stim1−/− platelets. Fura-2–loaded wild-type (black line) or Stim1−/− (gray line) platelets were stimulated with 0.1 U/ml thrombin, 10 μM ADP, or 10 μg/ml CRP in the presence of extracellular 1 mM EGTA or 0.5 mM Ca2+, and [Ca2+]i was monitored. Representative measurements (A) and maximal increase in intracellular Ca2+ concentrations compared with baseline levels before stimulus (Δ[Ca 2+]i) ± SD (n = 4 mice per group; B) are shown. (C) Impaired aggregation of Stim1−/− platelets (gray lines) in response to CRP and collagen but not ADP and thrombin (recording time = 10 min). (D) Flow cytometric analysis of integrin αIIbβ3 activation (binding of JON/A-PE; left) and degranulation-dependent P-selectin exposure (right) in response to 0.1 U/ml thrombin, 10 μM ADP, 10 μg/ml CRP, and 1 μg/ml convulxin. Results are means ± SD (n = 6 mice per group). (E) Stim1−/− platelets in whole blood fail to form stable thrombi when perfused over a collagen-coated (0.2 mg/ml) surface at a shear rate of 1,700 s−1. (top) Representative phase-contrast images. (bottom) Mean surface coverage (left) and relative platelet deposition as measured by the integrated fluorescent intensity per square millimeter (right) ± SD (n = 4 mice). ***, P < 0.001. Bar, 100 μm.
Figure 3.
In vivo analysis of thrombosis and hemostasis. (A–C) Mesenteric arterioles were treated with FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo video microscopy. Representative images (A), the time to appearance of the first thrombus >20 μm (B), and the time to vessel occlusion (C) are shown. Each symbol represents one individual. The asterisk in A indicates occlusion of the vessel. Horizontal bars in B represent means. Bar, 50 μm. (D and E) The abdominal aorta was mechanically injured, and blood flow was monitored for 30 min or until complete occlusion occurred (blood flow stopped >5 min). (D) Representative cross sections of the abdominal aorta of mice with wild-type or Stim1−/− platelets 30 min after injury. Bar, 250 μm. (E) Time to vessel occlusion. Each symbol represents one individual. (F) Tail bleeding times in wild-type and Stim1−/− chimeras. Each symbol represents one individual. Videos 1 and 2 are available at
http://www.jem.org/cgi/content/full/jem.20080302/DC1
.
Figure 4.
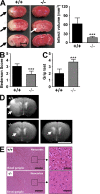
Stim1−/− chimeras are protected from cerebral ischemia. (A, left) Representative images of three corresponding coronal sections from control mice and Stim1−/− chimeras stained with TTC 24 h after tMCAO. Infarcts in Stim1−/− chimeras are restricted to the basal ganglia (white arrow), in contrast to controls (black arrows). (right) Brain infarct volumes in controls (n = 7) and Stim1−/− chimeras (n = 7). Values are mean ± SD. ***, P < 0.0001. Bar, 5 mm. (B and C) Neurological Bederson score and grip test assessed at day 1 after tMACO of controls (n = 7) and Stim1−/− chimeras (n = 7). Values are mean ± SD. ***, P < 0.0001. (D) The coronal T2-w MR brain image shows a large hyperintense ischemic lesion at day 1 after tMCAO in controls (white arrows; top left). Infarcts are smaller in Stim1−/− chimeras (white arrow; bottom left), and T2 hyperintensity decreases by day 7 during infarct maturation (white arrow; bottom right). Importantly, hypointense areas indicating intracerebral hemorrhage were not seen in Stim1−/− chimeras, demonstrating that Stim1 deficiency does not increase the risk of hemorrhagic transformation, even at advanced stages of infarct development. Bar, 5 mm. (E) Hematoxylin and eosin–stained sections of corresponding territories in the ischemic hemispheres of control and Stim1−/− chimeras. Infarcts are restricted to the basal ganglia in Stim1−/− chimeras but consistently include the cortex in controls. Bars: (left) 200 μm; (right) 50 μm.
Similar articles
- Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation.
Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bösl M, Stoll G, Nieswandt B. Braun A, et al. Blood. 2009 Feb 26;113(9):2056-63. doi: 10.1182/blood-2008-07-171611. Epub 2008 Oct 2. Blood. 2009. PMID: 18832659 - Roles of platelet STIM1 and Orai1 in glycoprotein VI- and thrombin-dependent procoagulant activity and thrombus formation.
Gilio K, van Kruchten R, Braun A, Berna-Erro A, Feijge MA, Stegner D, van der Meijden PE, Kuijpers MJ, Varga-Szabo D, Heemskerk JW, Nieswandt B. Gilio K, et al. J Biol Chem. 2010 Jul 30;285(31):23629-38. doi: 10.1074/jbc.M110.108696. Epub 2010 Jun 2. J Biol Chem. 2010. PMID: 20519511 Free PMC article. - An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice.
Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Mühlstedt S, Zorn C, Huber M, Schmittwolf C, Jagla W, Yu P, Kerkau T, Schulze H, Nehls M, Nieswandt B. Grosse J, et al. J Clin Invest. 2007 Nov;117(11):3540-50. doi: 10.1172/JCI32312. J Clin Invest. 2007. PMID: 17965774 Free PMC article. - STIM and Orai in platelet function.
Varga-Szabo D, Braun A, Nieswandt B. Varga-Szabo D, et al. Cell Calcium. 2011 Sep;50(3):270-8. doi: 10.1016/j.ceca.2011.04.002. Epub 2011 May 25. Cell Calcium. 2011. PMID: 21616531 Review. - Molecular physiology and pathophysiology of stromal interaction molecules.
Nelson HA, Roe MW. Nelson HA, et al. Exp Biol Med (Maywood). 2018 Mar;243(5):451-472. doi: 10.1177/1535370218754524. Epub 2018 Jan 24. Exp Biol Med (Maywood). 2018. PMID: 29363328 Free PMC article. Review.
Cited by
- Emerging roles of store-operated Ca²⁺ entry through STIM and ORAI proteins in immunity, hemostasis and cancer.
Bergmeier W, Weidinger C, Zee I, Feske S. Bergmeier W, et al. Channels (Austin). 2013 Sep-Oct;7(5):379-91. doi: 10.4161/chan.24302. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511024 Free PMC article. Review. - Mutations of the Ca2+-sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1.
Kilch T, Alansary D, Peglow M, Dörr K, Rychkov G, Rieger H, Peinelt C, Niemeyer BA. Kilch T, et al. J Biol Chem. 2013 Jan 18;288(3):1653-64. doi: 10.1074/jbc.M112.417246. Epub 2012 Dec 4. J Biol Chem. 2013. PMID: 23212906 Free PMC article. - Ion Channels and Transporters in Muscle Cell Differentiation.
Chen L, Hassani Nia F, Stauber T. Chen L, et al. Int J Mol Sci. 2021 Dec 19;22(24):13615. doi: 10.3390/ijms222413615. Int J Mol Sci. 2021. PMID: 34948411 Free PMC article. Review. - R93W mutation in Orai1 causes impaired calcium influx in platelets.
Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. Bergmeier W, et al. Blood. 2009 Jan 15;113(3):675-8. doi: 10.1182/blood-2008-08-174516. Epub 2008 Oct 24. Blood. 2009. PMID: 18952890 Free PMC article. - Rac1 is essential for phospholipase C-gamma2 activation in platelets.
Pleines I, Elvers M, Strehl A, Pozgajova M, Varga-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C, Nieswandt B. Pleines I, et al. Pflugers Arch. 2009 Mar;457(5):1173-85. doi: 10.1007/s00424-008-0573-7. Epub 2008 Aug 13. Pflugers Arch. 2009. PMID: 18704487
References
- Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. - PubMed
- Bhatt, D.L., and E.J. Topol. 2003. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2:15–28. - PubMed
- Kleinschnitz, C., M. Pozgajova, M. Pham, M. Bendszus, B. Nieswandt, and G. Stoll. 2007. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 115:2323–2330. - PubMed
- Sachs, U.J., and B. Nieswandt. 2007. In vivo thrombus formation in murine models. Circ. Res. 100:979–991. - PubMed
- Berridge, M.J., M.D. Bootman, and H.L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous