A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis - PubMed (original) (raw)
. 2008 Jun 15;68(12):4727-35.
doi: 10.1158/0008-5472.CAN-07-6499.
Sonia L Hernandez, Indranil Das, Audrey Ahn, Jianzhong Huang, Marina Vorontchikhina, Anshula Sharma, Emi Kanamaru, Valeriya Borisenko, Dinuka M Desilva, Akihiko Suzuki, Xing Wang, Carrie J Shawber, Jessica J Kandel, Darrell J Yamashiro, Jan Kitajewski
Affiliations
- PMID: 18559519
- PMCID: PMC3690602
- DOI: 10.1158/0008-5472.CAN-07-6499
A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis
Yasuhiro Funahashi et al. Cancer Res. 2008.
Abstract
Notch signaling is required for vascular development and tumor angiogenesis. Although inhibition of the Notch ligand Delta-like 4 can restrict tumor growth and disrupt neovasculature, the effect of inhibiting Notch receptor function on angiogenesis has yet to be defined. In this study, we generated a soluble form of the Notch1 receptor (Notch1 decoy) and assessed its effect on angiogenesis in vitro and in vivo. Notch1 decoy expression reduced signaling stimulated by the binding of three distinct Notch ligands to Notch1 and inhibited morphogenesis of endothelial cells overexpressing Notch4. Thus, Notch1 decoy functioned as an antagonist of ligand-dependent Notch signaling. In mice, Notch1 decoy also inhibited vascular endothelial growth factor-induced angiogenesis in skin, establishing a role for Notch receptor function in this process. We tested the effects of Notch1 decoy on tumor angiogenesis using two models: mouse mammary Mm5MT cells overexpressing fibroblast growth factor 4 (Mm5MT-FGF4) and NGP human neuroblastoma cells. Exogenously expressed FGF4 induced Notch ligand expression in Mm5MT cells and xenografts. Notch1 decoy expression did not affect tumorigenicity of Mm5MT-FGF4 cells in vitro but restricted Mm5MT-FGF4 xenograft growth in mice while markedly impairing neoangiogenesis. Similarly, Notch1 decoy expression did not affect NGP cells in vitro but disrupted vessels and decreased tumor viability in vivo. These results strongly suggest that Notch receptor signaling is required for tumor neoangiogenesis and provides a new target for tumor therapy.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing financial interest in this work.
Figures
Figure 1
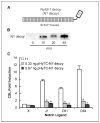
Notch1 decoy inhibits activation of Notch signaling stimulated by Notch ligands. A, schematic of Notch1 decoy containing the 36 endothelial growth factor repeats of rat Notch1 fused to human Fc. B, Western blotting to detect secreted Notch1 decoy in conditioned medium from HUVECs transduced with Ad-Notch1 decoy at indicated m.o.i. Bar, 100 μm. C, Notch1 decoy inhibits ligand-induced CSL reporter activity in coculture signaling assay. Activation of Notch signaling was measured in HeLa cells expressing Notch1 cocultured with 293 cells expressing Notch ligands. Columns, mean; bars, SD. *, P < 0.05.
Figure 2
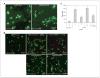
Notch1 decoy or compound E blocks Notch4-mediated HUVEC extensions. A, ectopic expression of Notch4 induces morphogenetic changes by HUVECs cultured on fibrin gel. HUVECs were transduced with Ad-Notch4 at 30 m.o.i. and Ad-GFP at 10 m.o.i. to mark-infected cells. Two days later, HUVEC transfectants were cocultured with transduced HUVECs on fibrin gel and morphologic changes were documented using fluorescence microscopy. Notch4-induced cell extensions (right, white arrows). B, Notch inhibition blocks Notch4-mediated HUVEC extensions. Notch4 expression induced cell extensions (top center) compared with control LacZ expressing HUVEC (top left), whereas treatment with 200 nmol/L compound E blocked Notch4-induced extensions (top right). Notch1 decoy expression blocks Notch4-induced cell extensions. Adenovirus-transduced HUVECs were cocultured on fibrin gels with stable HUVEC transfectants expressing either Fc (bottom left) or Notch1 decoy (bottom right) and photographed 2 d later. Bar, 200 μm. C, quantification of effect of Notch signal inhibition on Notch4-induced extensions. Reduction in extensions was statistically significant after treatment with compound E and expression of Notch1 decoy (P < 0.0001, both). Columns, mean; bars, SD.
Figure 3
Role of Notch signaling in VEGF-dependent in vivo angiogenesis. Inhibition of KP1/VEGF-induced angiogenesis with Notch1 decoy in mouse DAS assay. Representative photographs. Top, subcutaneous VEGF-induced angiogenesis with control COL1 (left) and KP1/VEGF cells transduced with GFP (middle) or with Notch1 decoy (right). Bottom, immunohistochemical analysis with CD31/PECAM antibody in muscle layer of skin (20×).
Figure 4
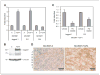
FGF4 induces the expression of Notch ligands in murine mammary carcinoma Mm5MT cells. Stable Mm5MT transfectants generated by retroviral gene transfer. A, quantitative RT-PCR analysis of the expression of Notch ligands showing induction of Jagged1 and Dll1 in Mm5MT-FGF4 compared with mock transfectants (Mm5MT-X). *, P < 0.05. B, Jagged1 protein is elevated in Mm5MT-FGF4 versus Mm5MT-X, as determined by Western blotting. C, reduction of Notch ligand expression in Mm5MT-FGF4 cells with PD166866, an inhibitor of FGFR kinase. *, P < 0.05. D, immunohistochemical analysis of Jagged1 staining in Mm5MT transfectants. Bar, 50 μm.
Figure 5
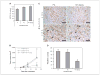
Notch1 decoy inhibits angiogenesis and s.c. tumor growth of Mm5MT-FGF4 tumors in mice. A, Mm5MT-FGF4 tumor growth in soft agar is little affected by expression of Fc or N1 decoy. B, tumor volumes of Mm5MT-FGF4-X and Mm5MT-FGF4-Fc differ significantly from Mm5MT-FGF4-Notch1 decoy transfectants in mice (**, day 21, P = 0.037 and P = 0.008, Mm5MT-FGF4-X and Mm5MT-FGF4-Fc versus Mm5MT-FGF4-Notch1 decoy, respectively). Points, mean; bars, SD. C, immunohistochemical analysis of neovessels with CD31/PECAM staining within day 21 tumors derived from Mm5MT-FGF4 transfectants. Top, bar, 100 μm; bottom, bar, 50 μm. D, quantitative analysis showed a reduction in CD31(+) neovessels in Mm5MT-FGF4-Notch1 decoy transfectants compared with Fc or mock-transfected tumors (*, P < 0.001 for both Mm5MT-FGF4-X and Mm5MT-FGF4-Fc versus Mm5MT-FGF4-Notch1 decoy). Columns, mean; bars, SD.
Figure 6
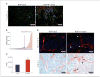
Notch1 decoy expression disrupts angiogenesis and impairs tumor viability in human NGP xenografts. We have previously reported that human neuroblastoma xenografts in mice have a mature, hierarchical vasculature that is relatively resistant to VEGF blockade (22). To determine whether Notch receptor activation contributed to NGP angiogenesis, we transfected NGP cells with the Notch1 decoy construct, which did not affect their ability to grow in culture (data not shown). There was, however, a marked decrease in tumor viability in vivo (A; red fluorescence, TUNEL; green fluorescence, erythrocytes; bar, 100 μm), with significantly increased fields that had high levels of tumor cell apoptosis [_B; P_ = 0.0002; TUNEL-positive cells in NGP-Notch1 decoy ( ) versus NGP-LacZ (
) versus NGP-LacZ ( ) tumors], and increased intratumoral hemorrhage [_C_; *, _P_ < 0.0001, quantitation of parenchymal erythrocyte signal, NGP-Notch1 decoy (
) tumors], and increased intratumoral hemorrhage [_C_; *, _P_ < 0.0001, quantitation of parenchymal erythrocyte signal, NGP-Notch1 decoy ( ) versus NGP-LacZ (
) versus NGP-LacZ ( ) tumors]. In addition, the tumor vessel networks in NGP-Notch1 decoy xenografts seemed to have been physically disrupted compared with NGP-LacZ controls, with immunostaining for ECs and VMCs (using anti-CD31/PECAM and αSMA antibodies, respectively) demonstrating lack of continuity of these vascular cell layers (D; bar, 50 μm). Individual vascular cells seemed detached from one another. Taken together, these results suggest that Notch1 decoy expression disrupted the ability of ECs and VMCs to form stable vascular conduits, causing vessel breakdown, hemorrhage, and ischemia of tumor tissues.
) tumors]. In addition, the tumor vessel networks in NGP-Notch1 decoy xenografts seemed to have been physically disrupted compared with NGP-LacZ controls, with immunostaining for ECs and VMCs (using anti-CD31/PECAM and αSMA antibodies, respectively) demonstrating lack of continuity of these vascular cell layers (D; bar, 50 μm). Individual vascular cells seemed detached from one another. Taken together, these results suggest that Notch1 decoy expression disrupted the ability of ECs and VMCs to form stable vascular conduits, causing vessel breakdown, hemorrhage, and ischemia of tumor tissues.
Similar articles
- Endothelial Jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1.
Pedrosa AR, Trindade A, Fernandes AC, Carvalho C, Gigante J, Tavares AT, Diéguez-Hurtado R, Yagita H, Adams RH, Duarte A. Pedrosa AR, et al. Arterioscler Thromb Vasc Biol. 2015 May;35(5):1134-46. doi: 10.1161/ATVBAHA.114.304741. Epub 2015 Mar 12. Arterioscler Thromb Vasc Biol. 2015. PMID: 25767274 - KSHV-induced notch components render endothelial and mural cell characteristics and cell survival.
Liu R, Li X, Tulpule A, Zhou Y, Scehnet JS, Zhang S, Lee JS, Chaudhary PM, Jung J, Gill PS. Liu R, et al. Blood. 2010 Jan 28;115(4):887-95. doi: 10.1182/blood-2009-08-236745. Epub 2009 Nov 24. Blood. 2010. PMID: 19965636 Free PMC article. - NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth.
Kangsamaksin T, Murtomaki A, Kofler NM, Cuervo H, Chaudhri RA, Tattersall IW, Rosenstiel PE, Shawber CJ, Kitajewski J. Kangsamaksin T, et al. Cancer Discov. 2015 Feb;5(2):182-97. doi: 10.1158/2159-8290.CD-14-0650. Epub 2014 Nov 11. Cancer Discov. 2015. PMID: 25387766 Free PMC article. - Notch signaling regulates tumor angiogenesis by diverse mechanisms.
Dufraine J, Funahashi Y, Kitajewski J. Dufraine J, et al. Oncogene. 2008 Sep 1;27(38):5132-7. doi: 10.1038/onc.2008.227. Oncogene. 2008. PMID: 18758482 Free PMC article. Review. - Ligand-dependent Notch signaling in vascular formation.
Kume T. Kume T. Adv Exp Med Biol. 2012;727:210-22. doi: 10.1007/978-1-4614-0899-4_16. Adv Exp Med Biol. 2012. PMID: 22399350 Review.
Cited by
- Inhibition of the Notch-Hey1 axis blocks embryonal rhabdomyosarcoma tumorigenesis.
Belyea BC, Naini S, Bentley RC, Linardic CM. Belyea BC, et al. Clin Cancer Res. 2011 Dec 1;17(23):7324-36. doi: 10.1158/1078-0432.CCR-11-1004. Epub 2011 Sep 23. Clin Cancer Res. 2011. PMID: 21948088 Free PMC article. - Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT.
De Francesco EM, Maggiolini M, Musti AM. De Francesco EM, et al. Int J Mol Sci. 2018 Jul 10;19(7):2011. doi: 10.3390/ijms19072011. Int J Mol Sci. 2018. PMID: 29996493 Free PMC article. Review. - MPDZ promotes DLL4-induced Notch signaling during angiogenesis.
Tetzlaff F, Adam MG, Feldner A, Moll I, Menuchin A, Rodriguez-Vita J, Sprinzak D, Fischer A. Tetzlaff F, et al. Elife. 2018 Apr 5;7:e32860. doi: 10.7554/eLife.32860. Elife. 2018. PMID: 29620522 Free PMC article. - New Approaches to Target T-ALL.
Roti G, Stegmaier K. Roti G, et al. Front Oncol. 2014 Jul 8;4:170. doi: 10.3389/fonc.2014.00170. eCollection 2014. Front Oncol. 2014. PMID: 25072021 Free PMC article. Review. - A new tumor suppressor role for the Notch pathway in bladder cancer.
Rampias T, Vgenopoulou P, Avgeris M, Polyzos A, Stravodimos K, Valavanis C, Scorilas A, Klinakis A. Rampias T, et al. Nat Med. 2014 Oct;20(10):1199-205. doi: 10.1038/nm.3678. Epub 2014 Sep 7. Nat Med. 2014. PMID: 25194568
References
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. - PubMed
- Jain R, Duda D, Clark J, Loeffler J. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. - PubMed
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–7. - PubMed
- Shawber C, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. Bioessays. 2004;26:225–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5K01DK744629/DK/NIDDK NIH HHS/United States
- R01 CA088951/CA/NCI NIH HHS/United States
- R01 CA100451/CA/NCI NIH HHS/United States
- R01 HL062454/HL/NHLBI NIH HHS/United States
- K01 DK074629/DK/NIDDK NIH HHS/United States
- 5R01HL62454/HL/NHLBI NIH HHS/United States
- K08 CA107077/CA/NCI NIH HHS/United States
- 5R01CA100451/CA/NCI NIH HHS/United States
- R01CA088951/CA/NCI NIH HHS/United States
- K08CA107077/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical