Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion - PubMed (original) (raw)
Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion
Yonghua Yang et al. Cancer Res. 2008.
Abstract
Heat shock protein (hsp) 90 is an ATP-dependent molecular chaperone that maintains the active conformation of client oncoproteins in cancer cells. An isoform, hsp90alpha, promotes extracellular maturation of matrix metalloproteinase (MMP)-2, involved in tumor invasion and metastasis. Knockdown of histone deacetylase (HDAC) 6, which deacetylates lysine residues in hsp90, induces reversible hyperacetylation and attenuates ATP binding and chaperone function of hsp90. Here, using mass spectrometry, we identified seven lysine residues in hsp90alpha that are hyperacetylated after treatment of eukaryotic cells with a pan-HDAC inhibitor that also inhibits HDAC6. Depending on the specific lysine residue in the middle domain involved, although acetylation affects ATP, cochaperone, and client protein binding to hsp90alpha, acetylation of all seven lysines increased the binding of hsp90alpha to 17-allyl-amino-demethoxy geldanamycin. Notably, after treatment with the pan-HDAC inhibitor panobinostat (LBH589), the extracellular hsp90alpha was hyperacetylated and it bound to MMP-2, which was associated with increased in vitro tumor cell invasiveness. Treatment with antiacetylated hsp90alpha antibody inhibited in vitro invasion by tumor cells. Thus, reversible hyperacetylation modulates the intracellular and extracellular chaperone function of hsp90, and targeting extracellular hyperacetylated hsp90alpha may undermine tumor invasion and metastasis.
Figures
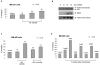
Figure 1. Treatment with pan-HDAC inhibitor induces acetylation of hsp90α, and p300 is the HAT for hsp90α
(A, B) HEK293 cells were transfected with either FLAG (F)-tagged hsp90α (3 μg) or empty vector pcDNA3 and treated with the indicated concentration of LBH589 (LBH) (A) or vorinostat (B) for 24 hours. Immunoprecipitates (IP) with anti-F (M2) antibody-conjugated beads were immunoblotted (IB) with either anti-rabbit acetyl lysine (AcK) or anti-rabbit FLAG (F) antibody. (C) p300 acts as a histone acetyl-transferase for hsp90α in vitro. The in vitro translated hsp90α was incubated with the combination of recombinant p300 and acetyl (Ac)-CoA followed by immunoblotting with anti-AcK antibody. The lane labeled ‘control’ contained LBH-induced acetylated F-hsp90α (D) p300 promotes acetylation of hsp90 in a dose dependent manner. HEK293 cells were co-transfected with F-hsp90α and the indicated amount of HA-tagged p300 (μg). Immunoprecipitates with anti-M2 antibody were immunoblotted with either anti-AcK or anti-F antibody. Immunoblots with anti-HA.11 or β-actin antibody served as loading control for p300 levels.
Figure 2. The individual K/R or K/Q substitutions do not affect the overall acetylation level of hsp90 but affect the ability of hsp90α to bind ATP, co-chaperones and client proteins
(A) The space-filling molecular structure model of hsp90. The seven lysine residues are shown in different colors. (B) Transfectants of F-hsp90α, with or without K/R substitutions, were treated with or without 100 nM of LBH. Following this, immunoprecipitates with M2 antibody were immunoblotted with either anti-AcK or anti-F antibody. (C, D) Transfectants of F-hsp90α with K/Q (C) but not K/R substitutions (D) affect ATP binding of hsp90α. Precipitates from the mixture of cell lysates containing hsp90α and ATP-sepharose were analyzed with anti-F antibody. Also, following transfections of F-hsp90α, with or without K/Q or K/R substitutions, immunoprecipitates with M2 antibody were immunoblotted with anti-CHIP, anti-p23, anti-hsp40, anti-hsp70, anti-c-Raf or anti-F antibody.
Figure 3. LBH589 (LBH)-induced acetylation increases B-GA and 17-AAG binding to hsp90α
(A) Individual K/Q substitutions increase hsp90α binding to biotinylated (B)-GA. Cell lysates from Figure 2D above were also incubated with B-GA followed by streptavidin coated agarose beads and eluted proteins were analyzed with anti-F antibody. (B) LBH589 increases hsp90 binding to GA and induces hsp90 acetylation dose-dependently. MB-468 cells cultured in DMEM medium containing 10 % FBS were treated with the indicated concentration of LBH589 for 16 hours. Following this, cell lysates were incubated with B-GA followed by streptavidin coated agarose beads and eluted proteins were analyzed with anti-hsp90α antibody. Acetylation and expression level of endogenous hsp90 were detected with anti-AcK and anti-hsp90 antibody, respectively. (C) LBH589 treatment preferentially increases hsp90α binding to 17-AAG. MB-468 cells ectopically expressing F-hsp90α were treated with 100 nM of LBH for 16 hours. Following this, equal amount of cell lysates were incubated with the indicated doses of 17-AAG for 30 min at 4°C, followed by incubation with B-GA and streptavidin-coated agarose beads. Precipitates from streptavidin coated beads were analyzed with anti-F antibody. (D) individual K/R substitution disrupted the affinity of LBH589-treated hsp90α for 17-AAG. Following treatment with either vehicle or 100 nM of LBH, cell lysates from HEK293 cells expressing F-hsp90 or K/R substitutions were incubated with vehicle or 50 nM of 17-AAG followed by incubation with B-GA and streptavidin coated agarose beads. Precipitates from streptavidin coated beads were analyzed with anti-F antibody.
Figure 4. Acetylation-dependent extra-cellular localization of hsp90α
(A) Serum-starvation of T47D breast cancer cells promotes extra-cellular localization of hsp90α. Concentrated extra-cellular medium or cell lysates from T47D cells, which were either serum-starved or cultured in 10 % FBS, were immunoblotted with anti-hsp90α antibody. β-actin served as a loading control. (A, right panel and B) Under starvation, both endogenous (4A, right panel) and exogenous hsp90α (B) are secreted from T47D cells in the acetylated form. Extra-cellular hsp90α was immunoprecipitated with anti-AcK antibody and immunoblotted with either anti-hsp90α or M2 antibody. (C) In serum-starved T47D cells, K/R substitutions at K69, K100 and K558 decrease, while K/Q substitutions increase the level of extracellular hsp90α. Supernatants of serum starved T47D cells transfected with the indicated F-tagged hsp90α mutant constructs were concentrated and immunoblotted with anti-F antibody. Coomassie-stained non-specific proteins served as the loading control. (D) K/Q substitution affects hsp90 expression on cell surface. MB-231 cells transfected with the indicated constructs were cultured under serum-free condition for 24 hours and followed by the labeling of surface protein with biotinylation. Biotinylated hsp90 on cell surface was detected with anti-F antibody. Biotinylated actin on cell surface served as loading control. Supernatants of serum-starved MB-231 cells transfected with the indicated F-tagged K/Q hsp90α mutant constructs were also concentrated and immunoprecipitates with anti-M2 conjugated beads were immunoblotted with anti-MMP-2 antibody.
Figure 5. Acetylation of hsp90α promotes in vitro invasion by breast cancer cells
(A and B) LBH promotes in vitro invasion of MB-231 cells, associated with extra-cellular location and binding of acetylated hsp90α to MMP-2. (A) MB-231 cells were treated with the indicated concentrations of LBH589 for 16 hours. Following this, an aliquot of the cells was used for determining in vitro matrigel invasion (see text for details) Columns, average results of three independent experiments; bars, SD. *, p < 0.05. (B) Alternatively, supernatant of serum starved LBH treated MDA-MB-231 cells were concentrated and immunoprecipitates with anti-hsp90α antibody were immunoblotted with anti-AcK, anti-MMP-2 or hsp90α antibody. (C) HDAC inhibitors promote in vitro invasion by MB-468 breast cancer cells. Serum-starved MB-468 cells were used for determining in vitro matrigel invasion in the indicated concentrations of LBH589 or vorinostat and then evaluated for in vitro invasion. Columns, average results of three independent experiments; bars, SD. *, p < 0.05. (D) Stable transfection of K/Q but not K/R substituted mutants promote in vitro invasion by MB-468 cells. Serum-starved MB-468 cells expressing either F-hsp90α, or K/Q or K/R substituted mutants were used for determining in vitro matrigel invasion. Columns, average results of three independent experiments; bars, SD. *, p < 0.05.
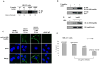
Figure 6. Anti-Ac-K69 antibody inhibits hsp90α-dependent in vitro invasion by breast cancer cells
(A) K/Q substitution at K69 promotes extracellular location of hsp90α. MB-231 cells transfected with either F-hsp90 or K69Q mutant were cultured under serum-free condition for 24 hours. Total extracellular and intracellular hsp90α were immunoprecipitated with anti-M2 conjugated beads and immunoblotted with anti-F antibody. The intensity of the bands was quantified using ImageQuant 5.2 software, and the ratio for intracellular to extracellular hsp90 is shown below the panel. Lane 1 is intracellular, and Lane 2 is extracellular. (B) Anti-Ac-K69 hsp90α antibody specifically recognizes acetylated form of both exogenous and endogenous hsp90α expressed in MB-231 cells. MB-231 cells were transfected with F-hsp90α followed by the treatment with LBH589. Immunoprecipitates of F-hsp90α with anti-M2 conjugated beads were immunoblotted with anti-K69-hsp90α antibody for the acetylation status and anti-F antibody for F-hsp90α expression. Cells transfected with empty vector followed the treatment with LBH589 served as control for specificity. Acetylation of endogenous hsp90α induced by LBH589 was also detected with anti-AcK69-hsp90α antibody (Lower panel). Immunoprecipitates of endogenous hsp90α with anti-hsp90α antibody from cell lysates of MB-231 cells treated with or without LBH589 were immunoblotted with either anti-AcK69-hsp90α or anti-hsp90α (rabbit) antibody. IgG served as the control for specificity of the immunoprecipitates. (C) LBH589 induces surface localization of acetylated hsp90α in MB-231 cells. Serum-starved MB-231 cells were treated with 40 nM LBH589 for 16 hours, followed by staining with anti-AcK69 hsp90α antibody and confocal microscopy. Cell cultured in RPMI with 10 % FBS and cells stained with rabbit IgG served as controls. (D) Inhibition of in vitro invasion by MB-231 cells by anti-AcK69 hsp90α antibody. Serum-starved MB-231 cells treated with 20 μg/mL anti-hsp90α or anti-AcK69 hsp90α antibody were used for determining in vitro matrigel invasion. Untreated cells, or cells treated with IgG were used as controls. Columns, average results of three independent experiments; bars, SD. *, p < 0.05.
Similar articles
- HDAC6 inhibition enhances 17-AAG--mediated abrogation of hsp90 chaperone function in human leukemia cells.
Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, Mandawat A, Atadja P, Bradner JE, Bhalla K. Rao R, et al. Blood. 2008 Sep 1;112(5):1886-93. doi: 10.1182/blood-2008-03-143644. Epub 2008 Jun 30. Blood. 2008. PMID: 18591380 - Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Bali P, et al. J Biol Chem. 2005 Jul 22;280(29):26729-34. doi: 10.1074/jbc.C500186200. Epub 2005 Jun 2. J Biol Chem. 2005. PMID: 15937340 - Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90.
Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, Bhalla K. Fiskus W, et al. Clin Cancer Res. 2007 Aug 15;13(16):4882-90. doi: 10.1158/1078-0432.CCR-06-3093. Clin Cancer Res. 2007. PMID: 17699868 - Organelle-specific Hsp90 inhibitors.
Seo YH. Seo YH. Arch Pharm Res. 2015 Sep;38(9):1582-90. doi: 10.1007/s12272-015-0636-1. Epub 2015 Jul 22. Arch Pharm Res. 2015. PMID: 26195286 Review. - Posttranslational modification and beyond: interplay between histone deacetylase 6 and heat-shock protein 90.
Liu P, Xiao J, Wang Y, Song X, Huang L, Ren Z, Kitazato K, Wang Y. Liu P, et al. Mol Med. 2021 Sep 16;27(1):110. doi: 10.1186/s10020-021-00375-3. Mol Med. 2021. PMID: 34530730 Free PMC article. Review.
Cited by
- Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents.
Yang H, Pinello CE, Luo J, Li D, Wang Y, Zhao LY, Jahn SC, Saldanha SA, Chase P, Planck J, Geary KR, Ma H, Law BK, Roush WR, Hodder P, Liao D. Yang H, et al. Mol Cancer Ther. 2013 May;12(5):610-20. doi: 10.1158/1535-7163.MCT-12-0930. Epub 2013 Apr 26. Mol Cancer Ther. 2013. PMID: 23625935 Free PMC article. - A phase I dose-escalation study of intravenous panobinostat in patients with lymphoma and solid tumors.
Sharma S, Beck J, Mita M, Paul S, Woo MM, Squier M, Gadbaw B, Prince HM. Sharma S, et al. Invest New Drugs. 2013 Aug;31(4):974-85. doi: 10.1007/s10637-013-9930-2. Epub 2013 Feb 2. Invest New Drugs. 2013. PMID: 23377661 Clinical Trial. - Hsp90 and p23 Molecular Chaperones Control Chromatin Architecture by Maintaining the Functional Pool of the RSC Chromatin Remodeler.
Echtenkamp FJ, Gvozdenov Z, Adkins NL, Zhang Y, Lynch-Day M, Watanabe S, Peterson CL, Freeman BC. Echtenkamp FJ, et al. Mol Cell. 2016 Dec 1;64(5):888-899. doi: 10.1016/j.molcel.2016.09.040. Epub 2016 Nov 3. Mol Cell. 2016. PMID: 27818141 Free PMC article. - Function of histone deacetylase 6 as a cofactor of nuclear receptor coregulator LCoR.
Palijan A, Fernandes I, Bastien Y, Tang L, Verway M, Kourelis M, Tavera-Mendoza LE, Li Z, Bourdeau V, Mader S, Yang XJ, White JH. Palijan A, et al. J Biol Chem. 2009 Oct 30;284(44):30264-74. doi: 10.1074/jbc.M109.045526. Epub 2009 Sep 10. J Biol Chem. 2009. PMID: 19744931 Free PMC article. - Silence of HDAC6 suppressed esophageal squamous cell carcinoma proliferation and migration by disrupting chaperone function of HSP90.
Tao H, Chen YY, Sun ZW, Chen HL, Chen M. Tao H, et al. J Cell Biochem. 2018 Aug;119(8):6623-6632. doi: 10.1002/jcb.26841. Epub 2018 Apr 17. J Cell Biochem. 2018. PMID: 29665050 Free PMC article. Retracted.
References
- Whitesell L, Lindquist SL. Hsp90 and the chaperoning of cancer. Nature Rev Cancer. 2005;5:761–72. - PubMed
- Isaacs J, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–17. - PubMed
- Panaretou B, Siligardi G, Meyer P, et al. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone Aha1. Molec Cell. 2002;10:1307–18. - PubMed
- Morishima Y, Kanelakis KC, Murphy PJ, et al. The hsp90 cochaperone p23 is the limiting component of the multiprotein hsp90/hsp70-based chaperone system in vivo where it acts to stabilize the client protein hsp90 complex. J Biol Chem. 2003;278:48754–63. - PubMed
- Roe S, Ali MM, Meyer P, et al. The mechanism of hsp90 regulation by the protein kinase-specific cochaperone p50cdc37. Cell. 2004;116:87–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous