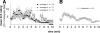Separate peripheral pathways for pruritus in man - PubMed (original) (raw)
Separate peripheral pathways for pruritus in man
Barbara Namer et al. J Neurophysiol. 2008 Oct.
Abstract
Recent findings suggest that itch produced by intradermal insertion of cowhage spicules in human is histamine independent. Neuronal mechanisms underlying nonhistaminergic itch are poorly understood. To investigate which nerve fibers mediate cowhage induced itch in man, action potentials were recorded from cutaneous C-fibers of the peroneal nerve in healthy volunteers using microneurography. Mechano-responsive and -insensitive C-nociceptors were tested for their responsiveness to cowhage spicules, histamine, and capsaicin. Cowhage spicules induced itching and activated all tested mechano-responsive C-units (24/24), but no mechano-insensitive C-fibers (0/17). Histamine also induced itch, but in contrast to cowhage, it caused lasting activation only in mechano-insensitive units (8/12). In mechano-responsive C-units, histamine caused no or only short and weak responses unrelated to the time course of itching. Capsaicin injections activated four of six mechano-responsive fibers and three of four mechano-insensitive C-fibers. Cowhage and histamine activate distinctly different nonoverlapping populations of C-fibers while inducing similar sensations of itch. We hypothesize that cowhage activates a pathway for itch that originates peripherally from superficial mechano-responsive (polymodal) C-fibers and perhaps other afferent units. It is distinct from the pathway for histamine-mediated pruritus and does not involve the histamine-sensitive mechano-insensitive fibers.
Figures
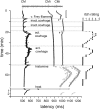
FIG. 1.
Specimen of a multifiber recording from 1 mechano-responsive (CM) and 2 mechano-insensitive nociceptors (CMi). A trace of the raw signal containing the C-fiber action potentials is shown on top. Conduction latencies of these three marked fibers (▪, ▵) in response to successive electrical stimulation at the receptive field are plotted from top to bottom. Top traces were recorded during stimulation with increasing frequencies (see □ on right side), followed by traces recorded during stimulation with mechanical stimuli (v. Frey filament), inactive (inact.) and active (act.) cowhage spicules, histamine ionthophoresis (histamine), and heat ( ). When activated by mechanical, chemical or heat test stimuli, C-fibers exhibit activity-dependent increase of response latency followed by a gradual normalization (“marking”). The mechano-responsive nociceptor is characterized by its moderate slowing to the initial repetitive electrical stimulation with increasing frequencies (0.125, 0.25, and 0.5 Hz; □) and the slowing in response to mechanical stimulation. The mechano-insensitive units are characterized my marked slowing during repetitive electrical stimulation and the lack of increase in latency during mechanical stimulation. The third fiber shows some “flip flopping” (marked with #) after the 1st mechanical stimulation resulting from action potential initiation in a different peripheral branches inside the receptive field. Flip flopping does not reflect activation. Note that the mechano-responsive fiber is activated during mechanical stimulation with the v.Frey filament and during application of inactive cowhage, but lasting activation is only seen after application of active cowhage. In contrast, the mechano-insensitive fibers do not respond to cowhage stimulation, but are active following histamine ionotophoresis. At the right side of the panel, the itch ratings of the subject, which were assessed during this experiment, are depicted. Ratings are given on a numerical rating scale from 0 (0 = no itch) to 10 (10 = maximal imaginable itch). Inactive cowhage does not evoke any itch, whereas active cowhage and histamine evoke itch similar in time course and maximum mirroring nicely the activation pattern of the fibers.
). When activated by mechanical, chemical or heat test stimuli, C-fibers exhibit activity-dependent increase of response latency followed by a gradual normalization (“marking”). The mechano-responsive nociceptor is characterized by its moderate slowing to the initial repetitive electrical stimulation with increasing frequencies (0.125, 0.25, and 0.5 Hz; □) and the slowing in response to mechanical stimulation. The mechano-insensitive units are characterized my marked slowing during repetitive electrical stimulation and the lack of increase in latency during mechanical stimulation. The third fiber shows some “flip flopping” (marked with #) after the 1st mechanical stimulation resulting from action potential initiation in a different peripheral branches inside the receptive field. Flip flopping does not reflect activation. Note that the mechano-responsive fiber is activated during mechanical stimulation with the v.Frey filament and during application of inactive cowhage, but lasting activation is only seen after application of active cowhage. In contrast, the mechano-insensitive fibers do not respond to cowhage stimulation, but are active following histamine ionotophoresis. At the right side of the panel, the itch ratings of the subject, which were assessed during this experiment, are depicted. Ratings are given on a numerical rating scale from 0 (0 = no itch) to 10 (10 = maximal imaginable itch). Inactive cowhage does not evoke any itch, whereas active cowhage and histamine evoke itch similar in time course and maximum mirroring nicely the activation pattern of the fibers.
FIG. 2.
Histogram of the number of activation periods induced by cowhage (top) and histamine stimulation (bottom) in mechano-responsive (□) and mechano-insensitive nociceptors (▪). For fibers with multiple cowhage applications, the maximum response was plotted. The number of activation periods induced by cowhage in mechano-responsive fibers shows a nearly Gaussian distribution. In contrast, mechano-insensitive fibers were unresponsive to cowhage application. The distribution of histamine responses in mechano-insensitive nociceptors reveals histamine responsive and unresponsive fibers. Mechano-sensitive afferents were largely unresponsive to histamine.
FIG. 3.
Specimen recordings from a mechano-responsive nociceptor during three successive cowhage stimulations (arrows 1, 2, 3). An original trace with the action potential of the fiber is plotted above the 1st stimulation. Note the bursting component of the response in repetition 2 and 3 and the weaker and somewhat delayed nonbursting pattern following the 1st application of cowhage.
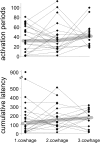
FIG. 4.
Responses of mechano-responsive nociceptors to 3 successive cowhage applications as measured by number of activation periods (top) and cumulative “marking” (bottom). Responses of the single units are linked, and median responses are marked with  . Note the considerable variation of the responses between and within the fibers; however, the median responses remain virtually constant.
. Note the considerable variation of the responses between and within the fibers; however, the median responses remain virtually constant.
FIG. 5.
Ratings (means ± SE) of cowhage- and histamine-induced itch sensations during microneurography. An open scale was used with the itch intensity of a mosquito bite arbitrarily rated as “10,” and itch sensation of double intensity to be rated as “20” and half the intensity as “5.” A: the 3 successive cowhage applications produced similar itch intensities with similar time course (cowhage 1–3). B: histamine ionthophoreses produced an itch sensation of similar intensity and duration (○).
Similar articles
- A role for nociceptive, myelinated nerve fibers in itch sensation.
Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, Shim B, LaMotte RH, Meyer RA. Ringkamp M, et al. J Neurosci. 2011 Oct 19;31(42):14841-9. doi: 10.1523/JNEUROSCI.3005-11.2011. J Neurosci. 2011. PMID: 22016517 Free PMC article. - A role for polymodal C-fiber afferents in nonhistaminergic itch.
Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. Johanek LM, et al. J Neurosci. 2008 Jul 23;28(30):7659-69. doi: 10.1523/JNEUROSCI.1760-08.2008. J Neurosci. 2008. PMID: 18650342 Free PMC article. - Microneurography of pruritus.
Handwerker HO. Handwerker HO. Neurosci Lett. 2010 Feb 19;470(3):193-6. doi: 10.1016/j.neulet.2009.06.092. Epub 2009 Jul 2. Neurosci Lett. 2010. PMID: 19576959 Review. - Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch.
Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Johanek LM, et al. J Neurosci. 2007 Jul 11;27(28):7490-7. doi: 10.1523/JNEUROSCI.1249-07.2007. J Neurosci. 2007. PMID: 17626210 Free PMC article. - Itch and Cough - Similar Role of Sensory Nerves in Their Pathogenesis.
Pecova T, Kocan I, Vysehradsky R, Pecova R. Pecova T, et al. Physiol Res. 2020 Mar 27;69(Suppl 1):S43-S54. doi: 10.33549/physiolres.934403. Physiol Res. 2020. PMID: 32228011 Free PMC article. Review.
Cited by
- Peripheral signaling pathways contributing to non-histaminergic itch in humans.
Fiebig A, Leibl V, Oostendorf D, Lukaschek S, Frömbgen J, Masoudi M, Kremer AE, Strupf M, Reeh P, Düll M, Namer B. Fiebig A, et al. J Transl Med. 2023 Dec 12;21(1):908. doi: 10.1186/s12967-023-04698-z. J Transl Med. 2023. PMID: 38087354 Free PMC article. - Transcutaneous Slowly Depolarizing Currents Elicit Pruritus in Patients with Atopic Dermatitis.
Rukwied R, Schnakenberg M, Solinkski HJ, Schmelz M, Weisshaar E. Rukwied R, et al. Acta Derm Venereol. 2020 Oct 28;100(17):adv00302. doi: 10.2340/00015555-3658. Acta Derm Venereol. 2020. PMID: 33026094 Free PMC article. - Recent advances in pruritus - what we have learned and where are we headed.
Yosipovitch G. Yosipovitch G. F1000 Med Rep. 2010 May 24;2:39. doi: 10.3410/M2-39. F1000 Med Rep. 2010. PMID: 20948846 Free PMC article. - A role for nociceptive, myelinated nerve fibers in itch sensation.
Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, Shim B, LaMotte RH, Meyer RA. Ringkamp M, et al. J Neurosci. 2011 Oct 19;31(42):14841-9. doi: 10.1523/JNEUROSCI.3005-11.2011. J Neurosci. 2011. PMID: 22016517 Free PMC article. - Itch signaling in the nervous system.
Jeffry J, Kim S, Chen ZF. Jeffry J, et al. Physiology (Bethesda). 2011 Aug;26(4):286-92. doi: 10.1152/physiol.00007.2011. Physiology (Bethesda). 2011. PMID: 21841076 Free PMC article. Review.
References
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72–77, 2001. - PubMed
- Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, LaMotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res 16: 291–298, 1999. - PubMed
- Cheigh NH Managing a common disorder in children: Atopic dermatitis. J Pediatr Health Care 17: 84–88, 2003. - PubMed
- Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science 265: 252–255, 1994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials